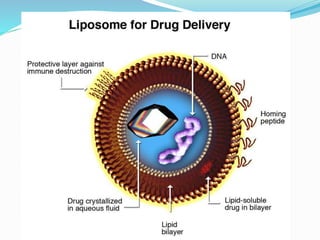
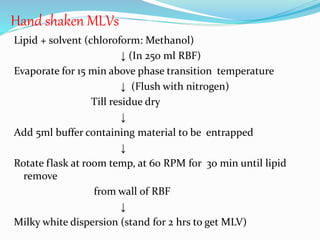
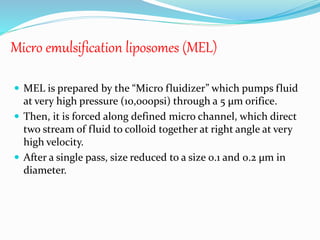
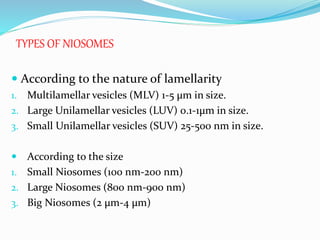
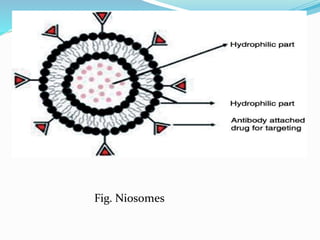
This document provides an overview of novel drug delivery systems (NDDS), including nanoparticles, liposomes, and niosomes. It classifies and defines each system. Nanoparticles are sub-nanosized structures that can encapsulate or attach drugs. Liposomes are bilayer vesicles that encapsulate drugs in an aqueous core. Niosomes are similar to liposomes but composed of non-ionic surfactants. The document discusses preparation methods, advantages and disadvantages, and applications of each delivery system to improve drug targeting and therapeutic effects.