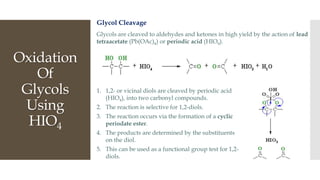
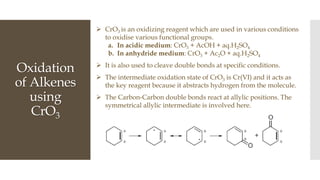
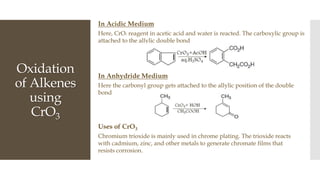
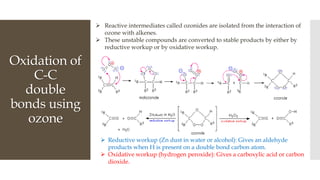
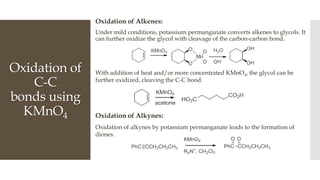
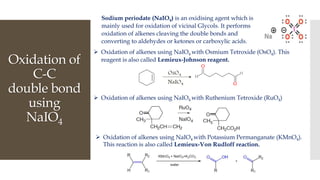
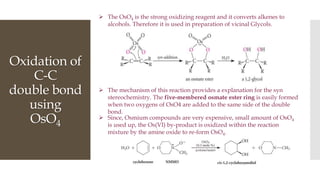
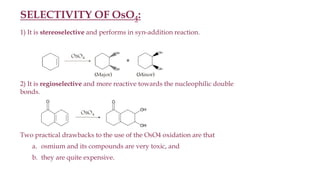
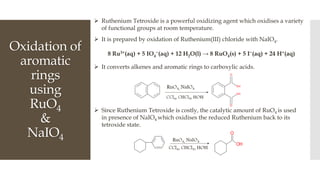
This document discusses various oxidation reactions involving carbon-carbon (C-C) bond cleavage, highlighting different reagents like periodic acid (HIO4), chromium trioxide (CrO3), ozone, potassium permanganate (KMnO4), sodium periodate (NAIO4), osmium tetroxide (OSO4), and ruthenium tetroxide (RuO4). Each reagent is examined for its specific mechanism, applications, and selectivity in converting alkenes, alkynes, glycols, and aromatic rings into carbonyl compounds and alcohols. The document also includes references to several organic chemistry textbooks for further reading.