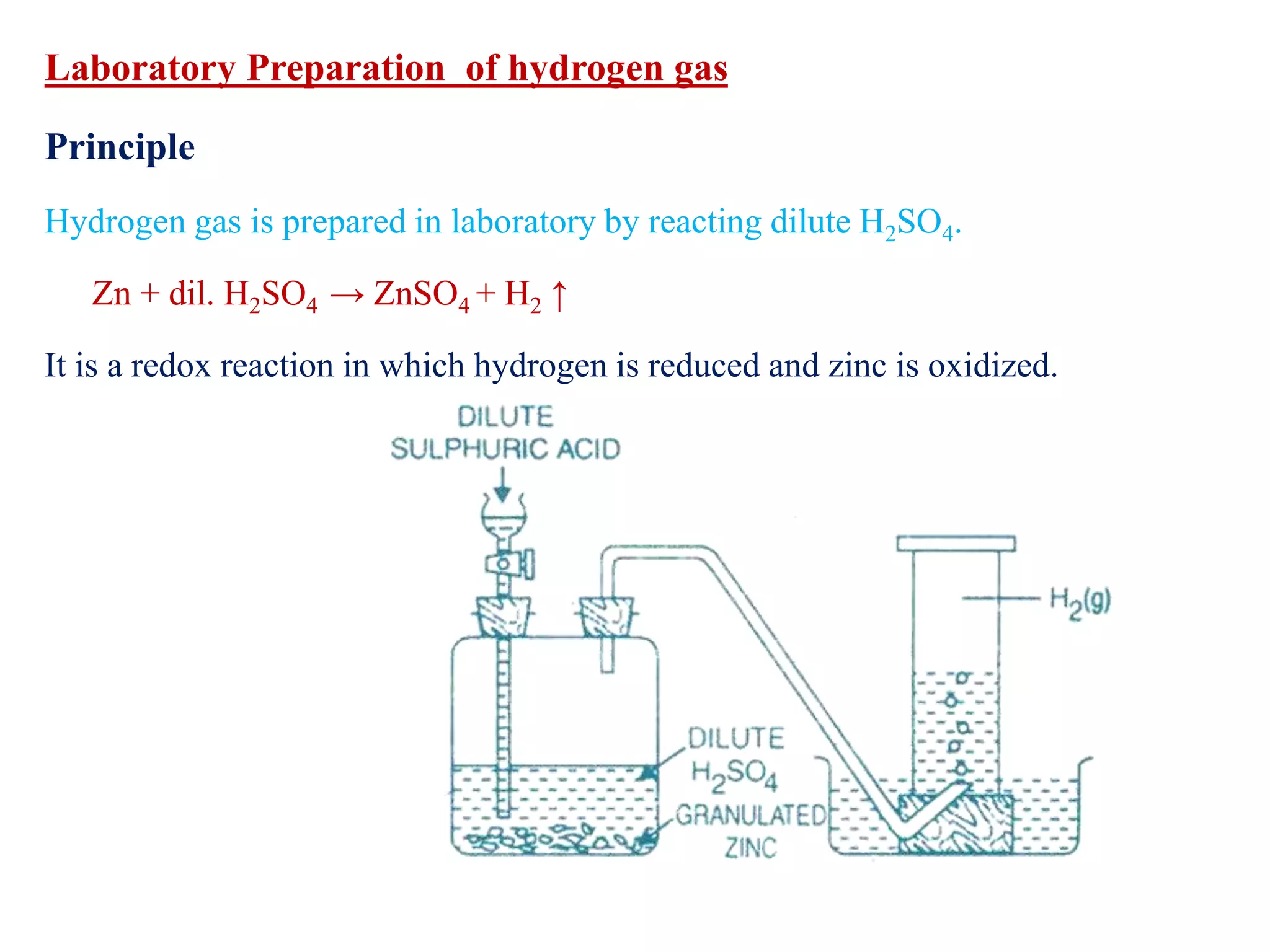
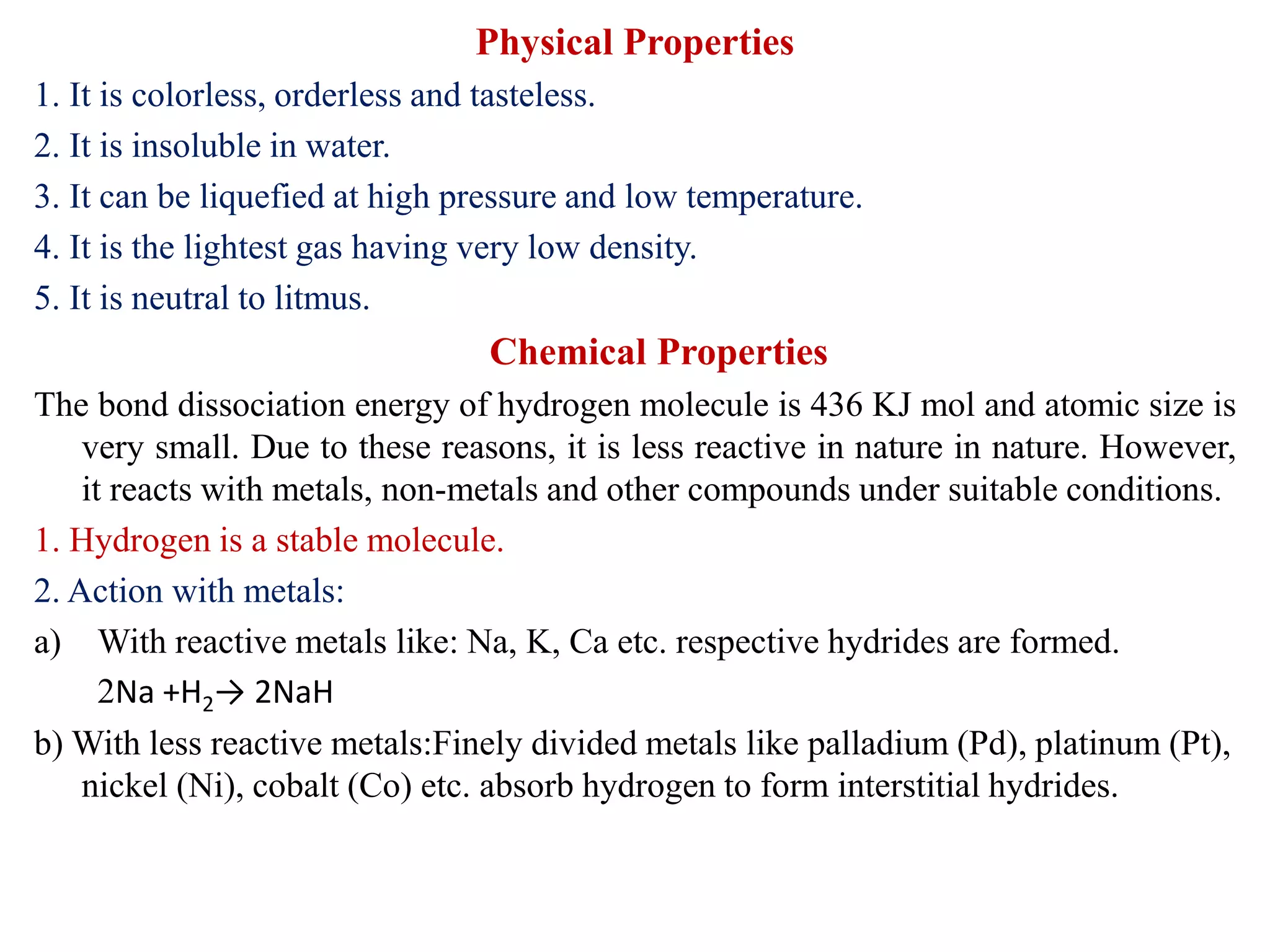
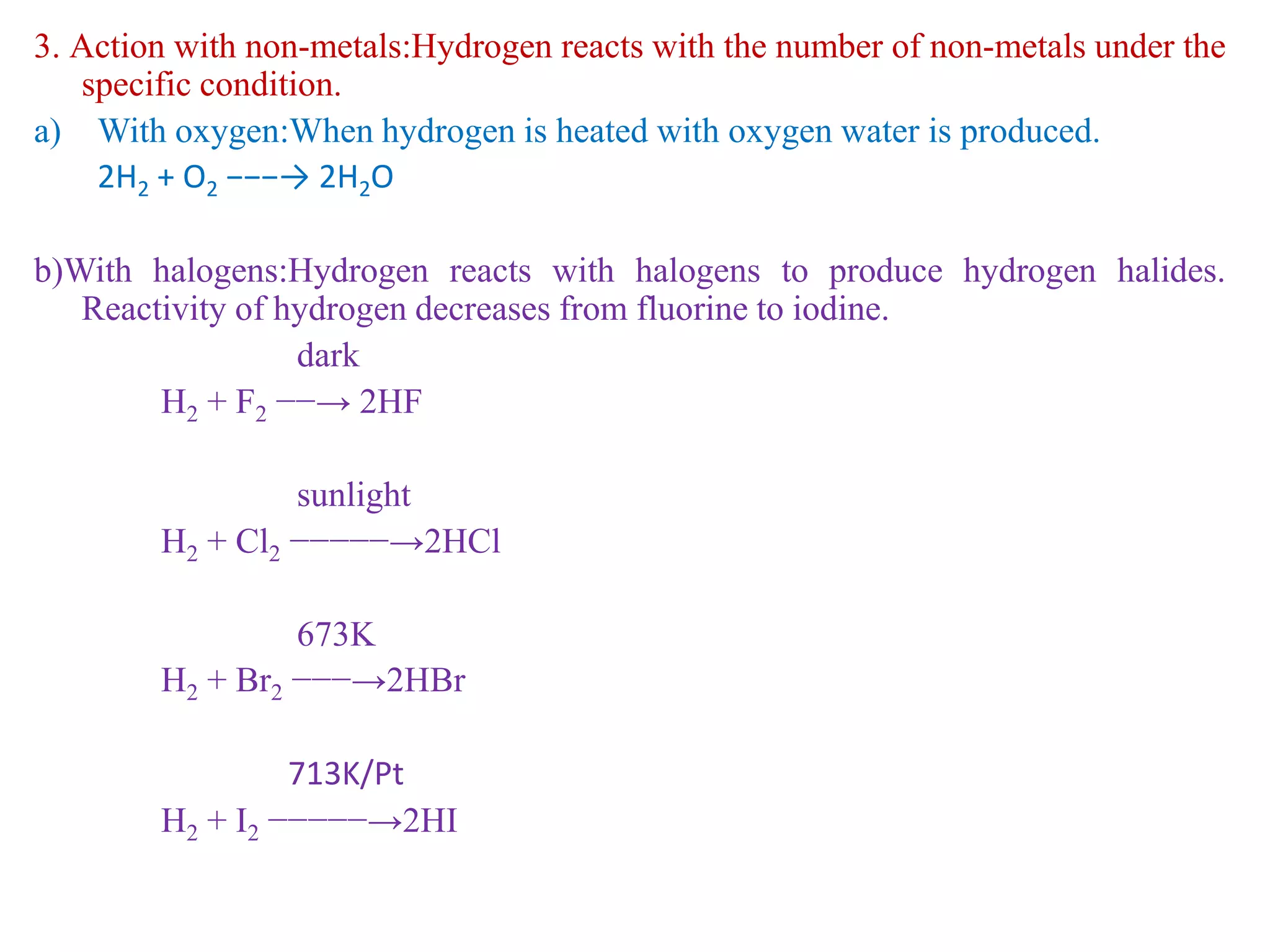
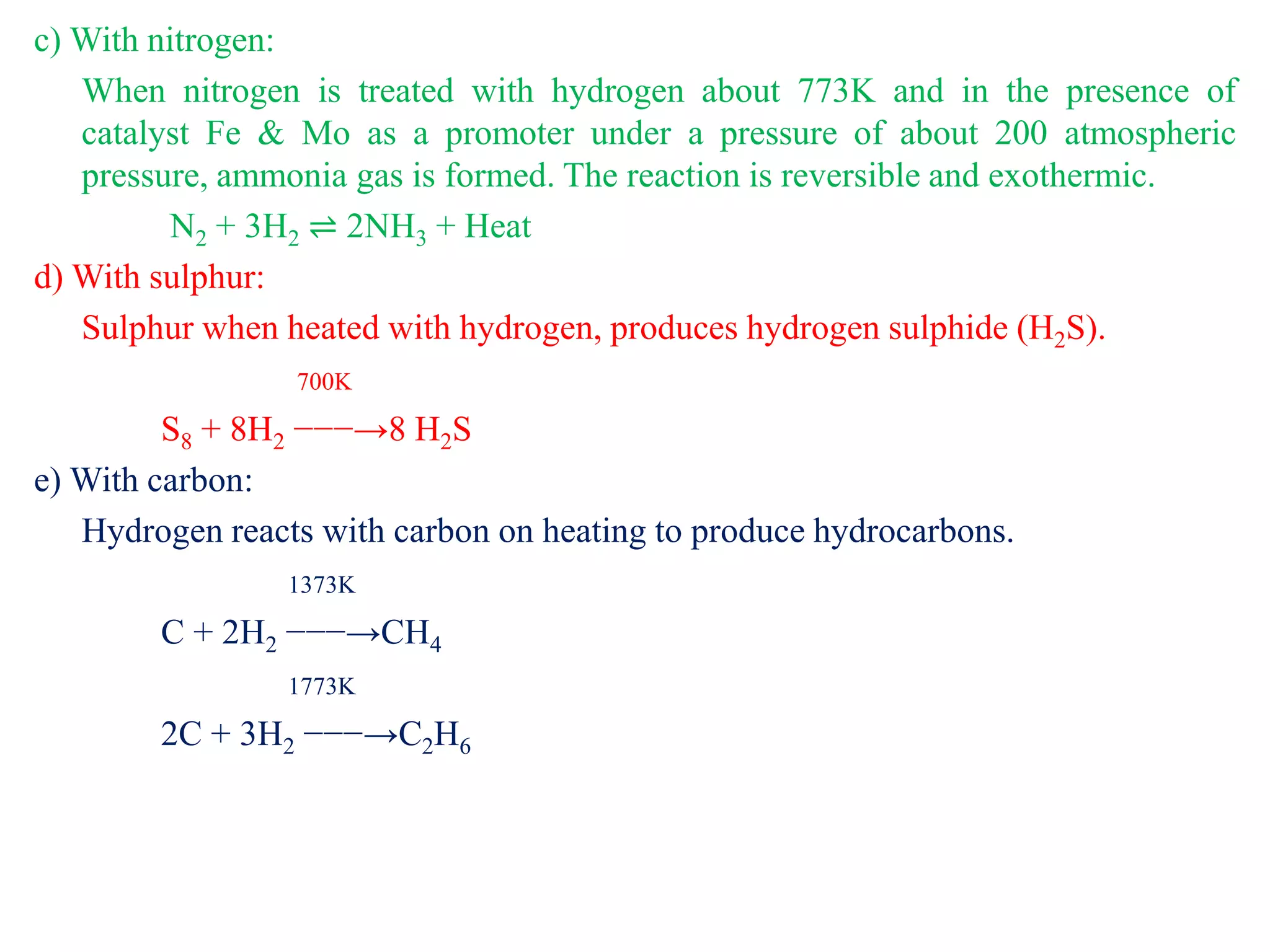
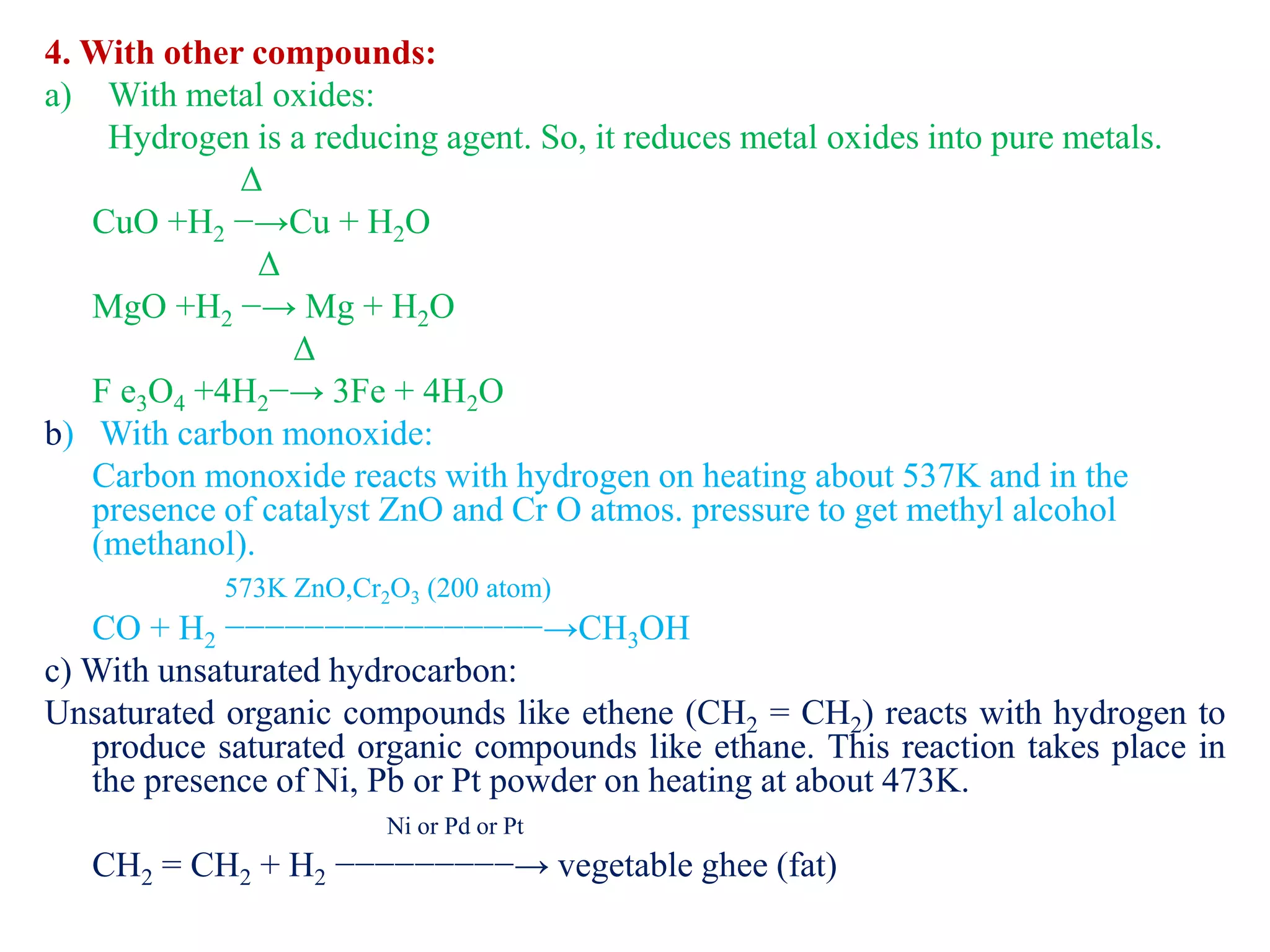
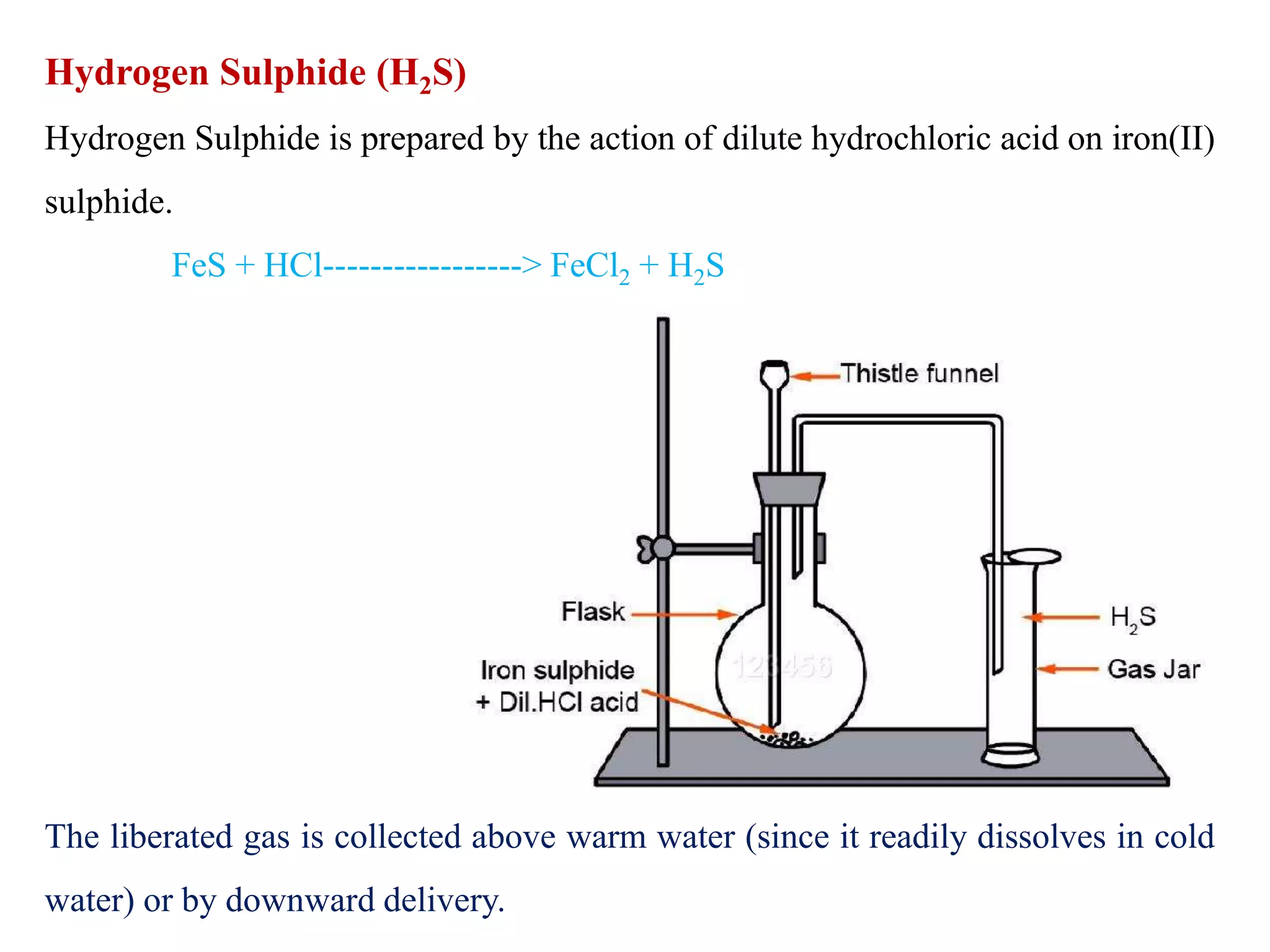
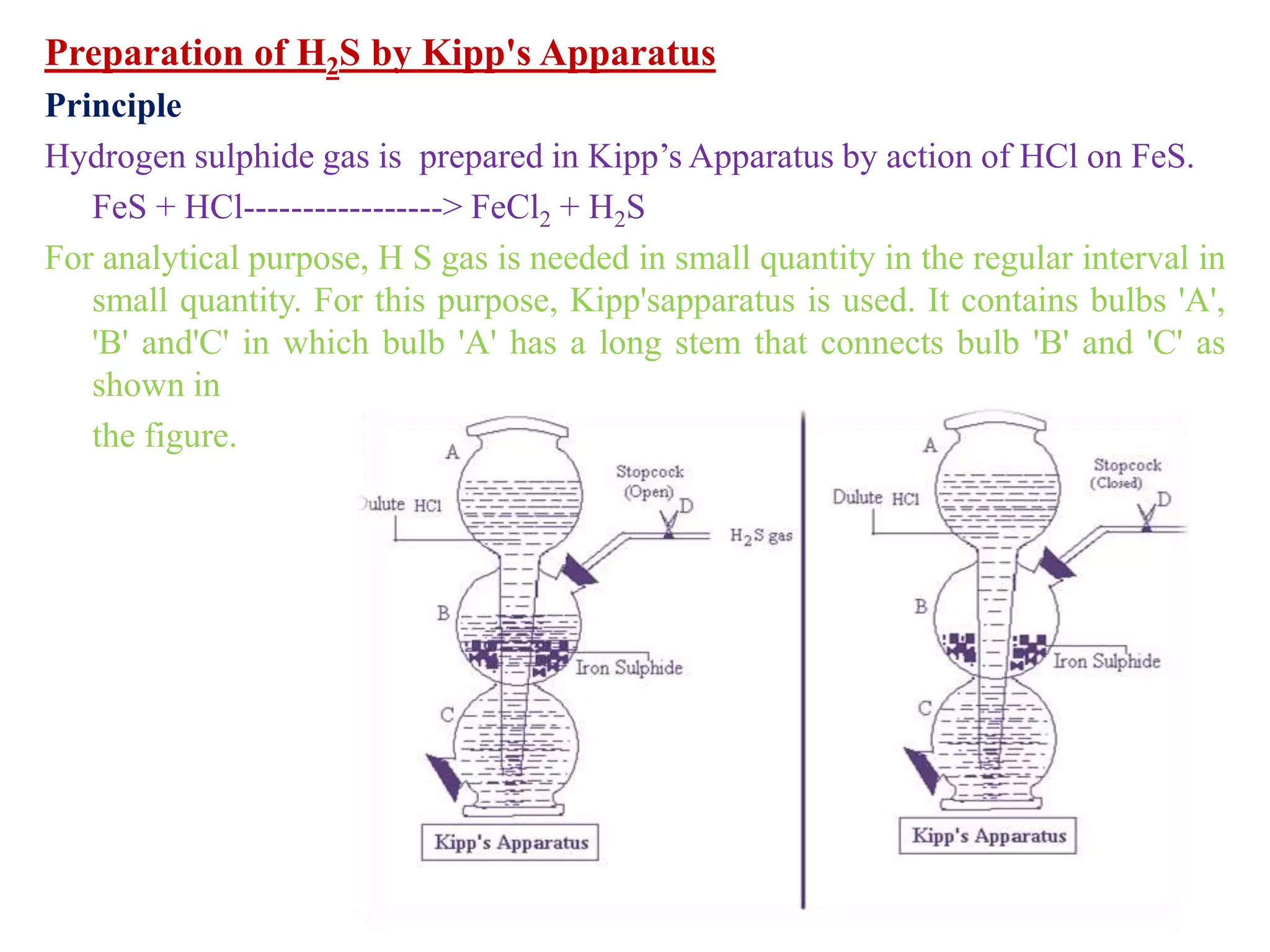
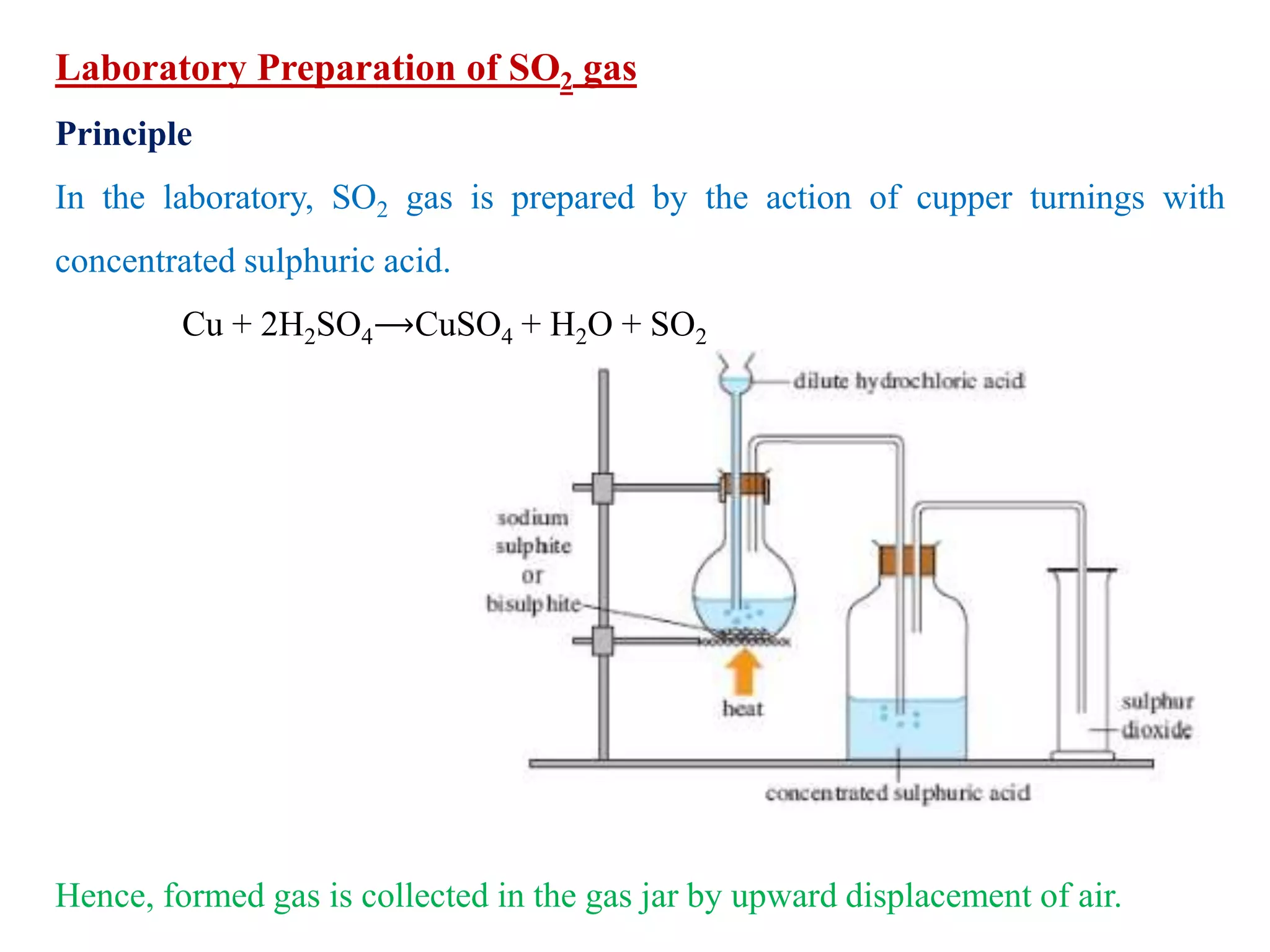
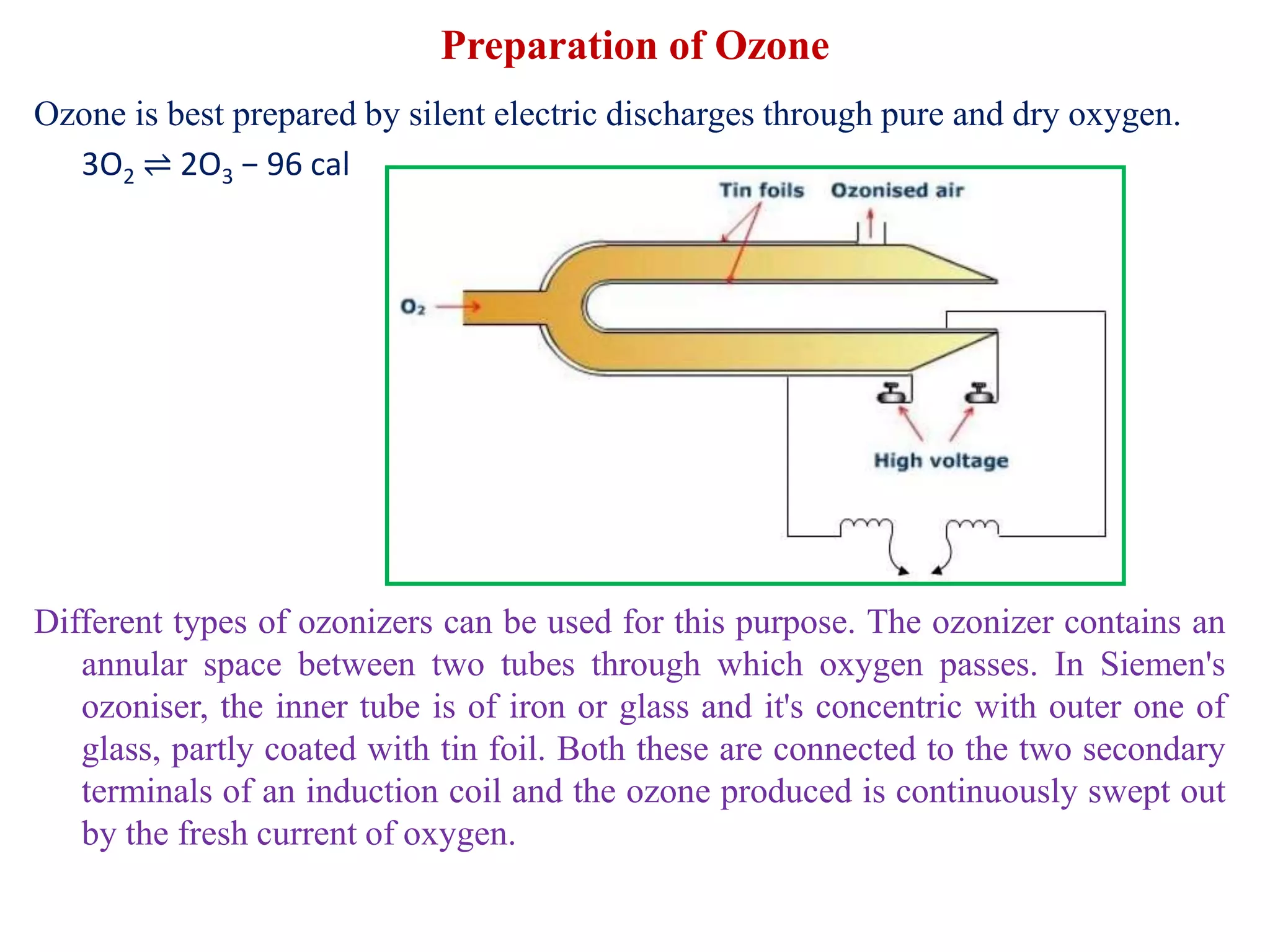
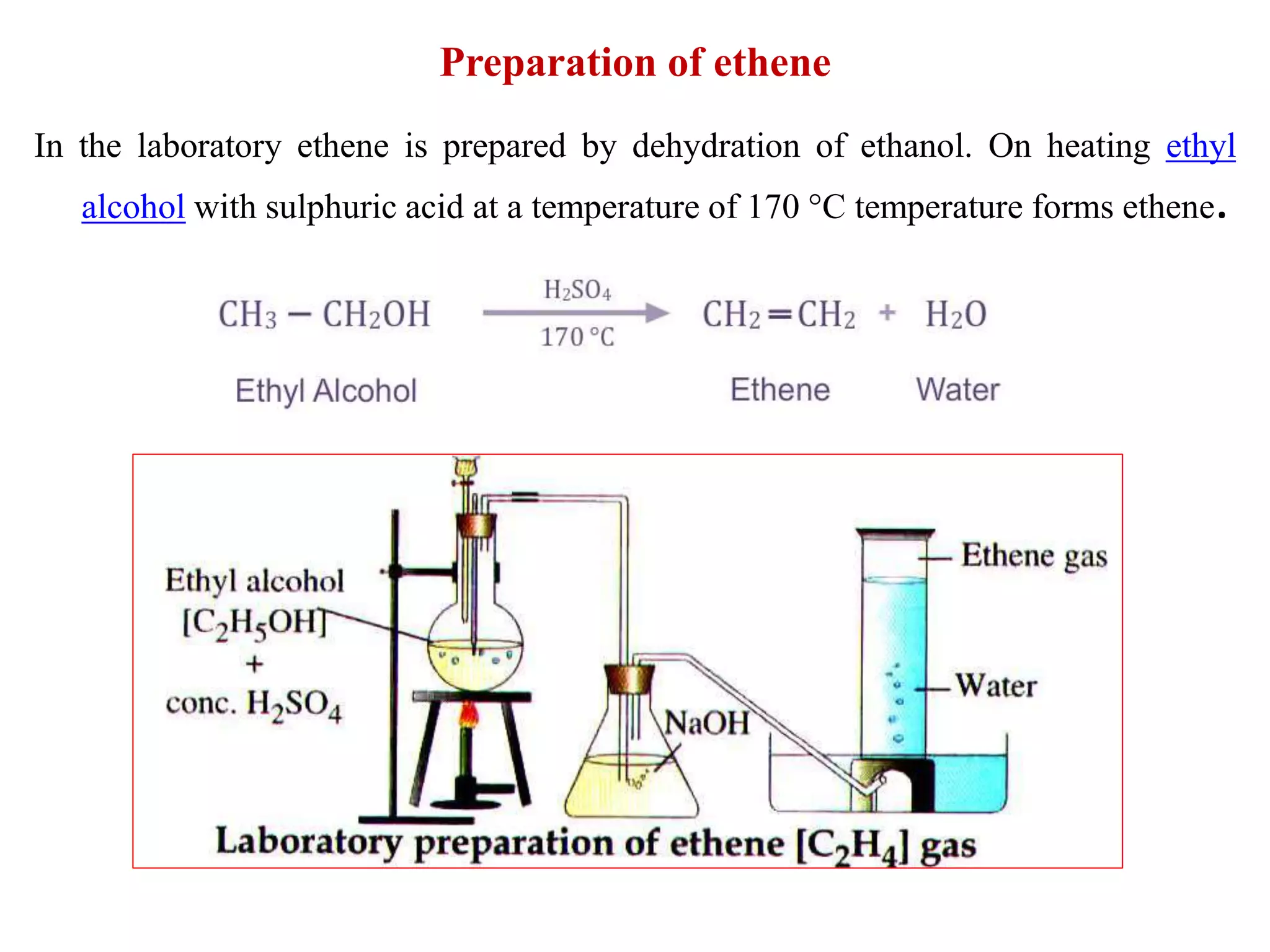
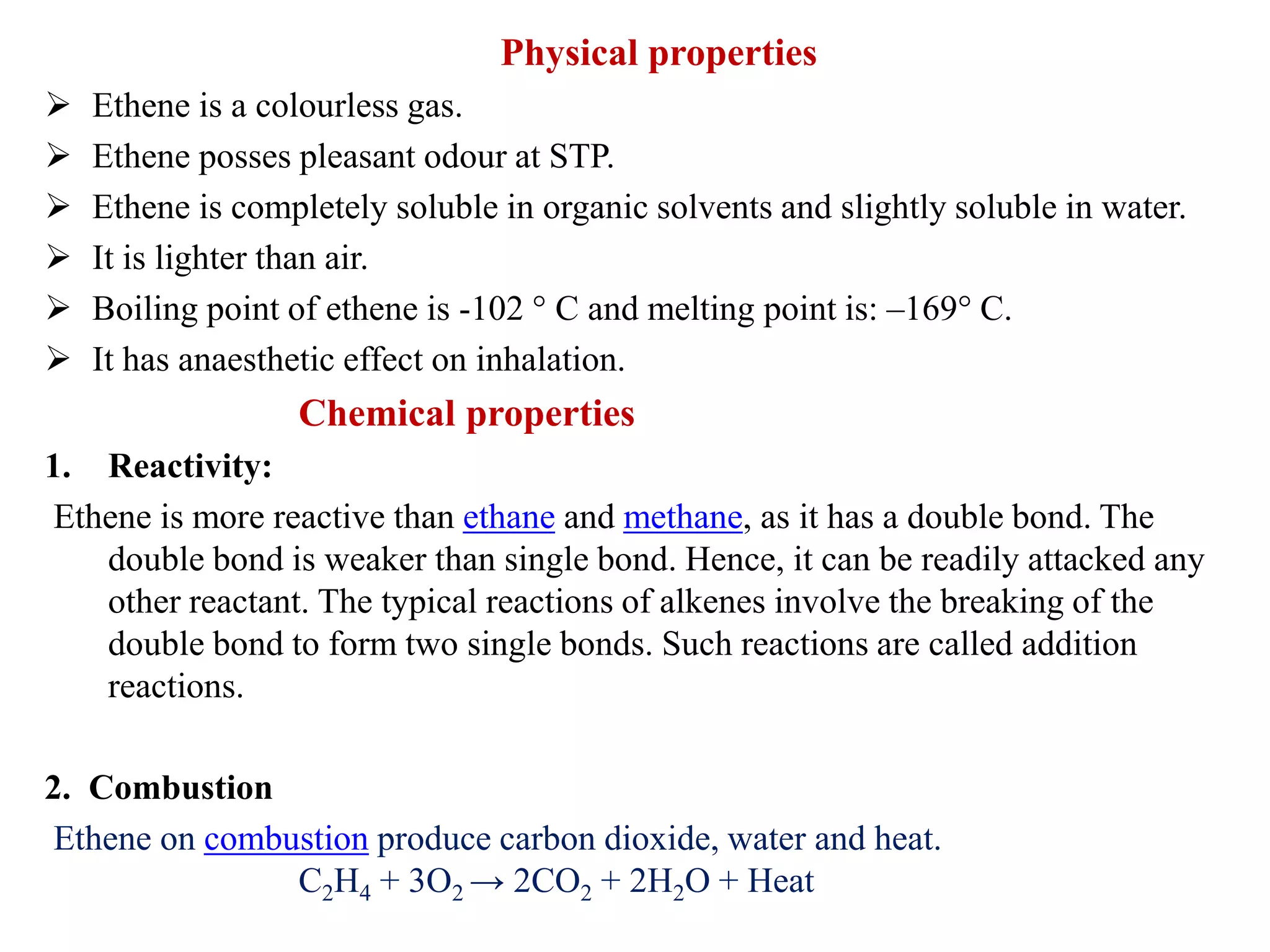
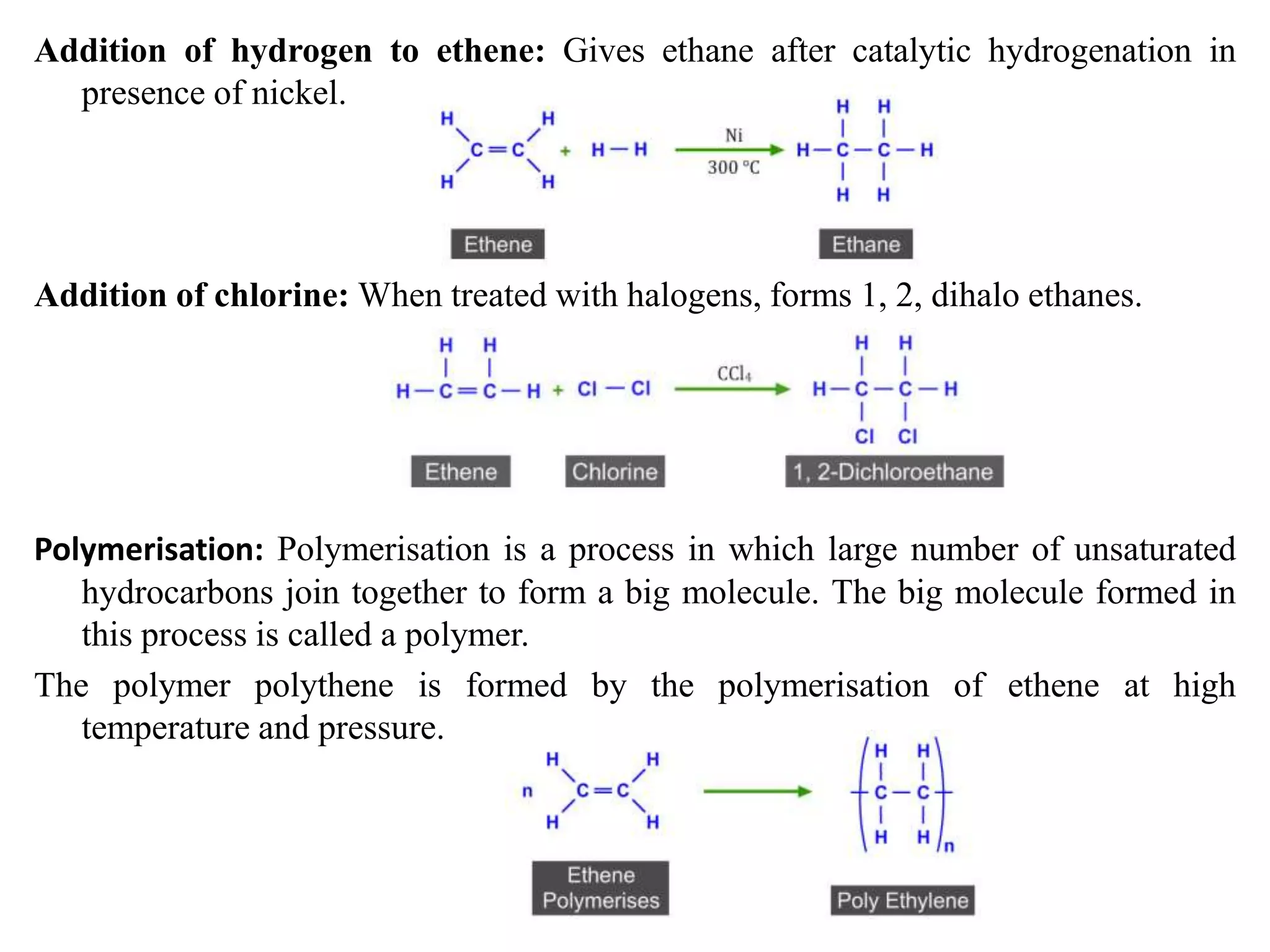
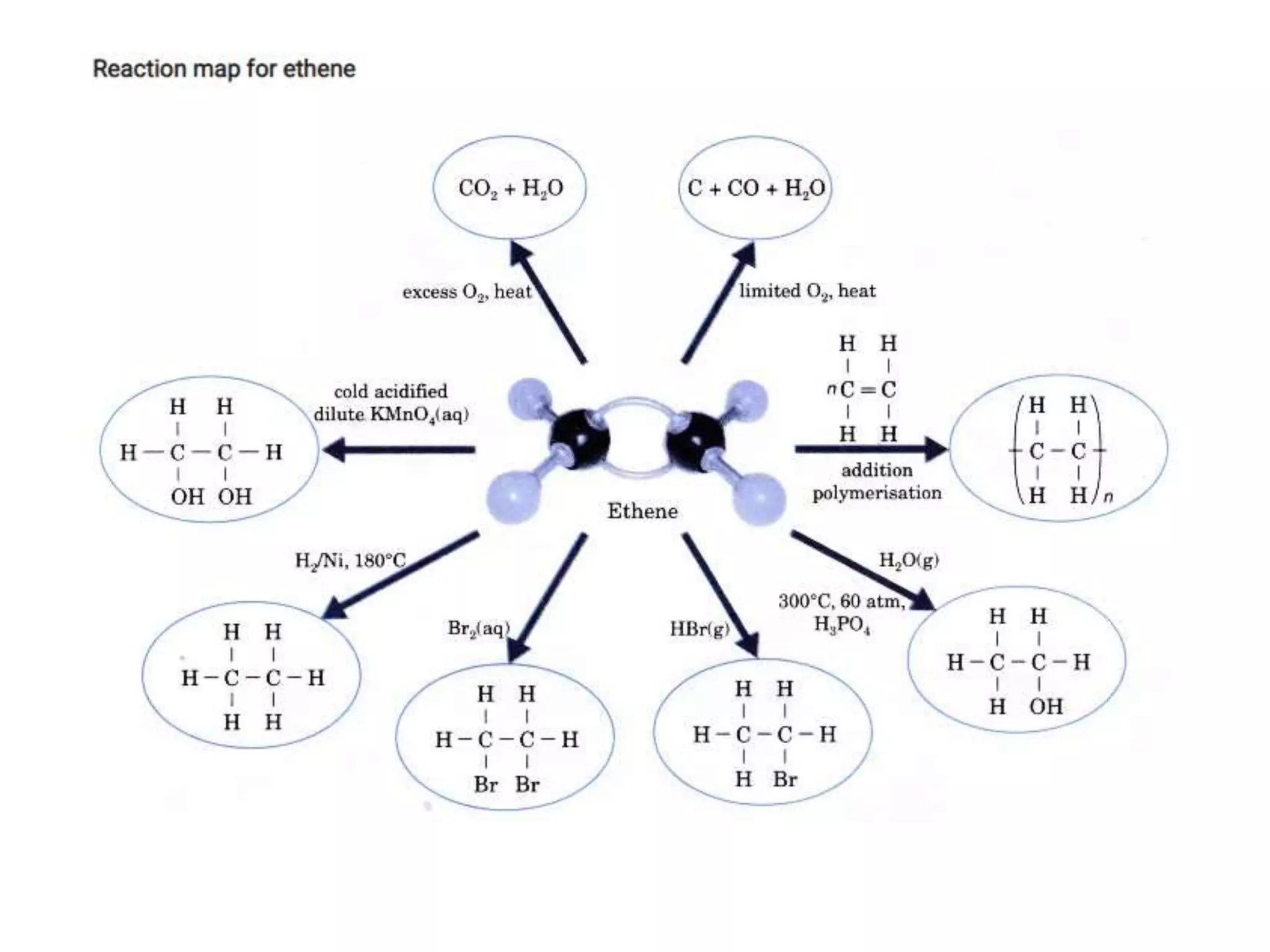
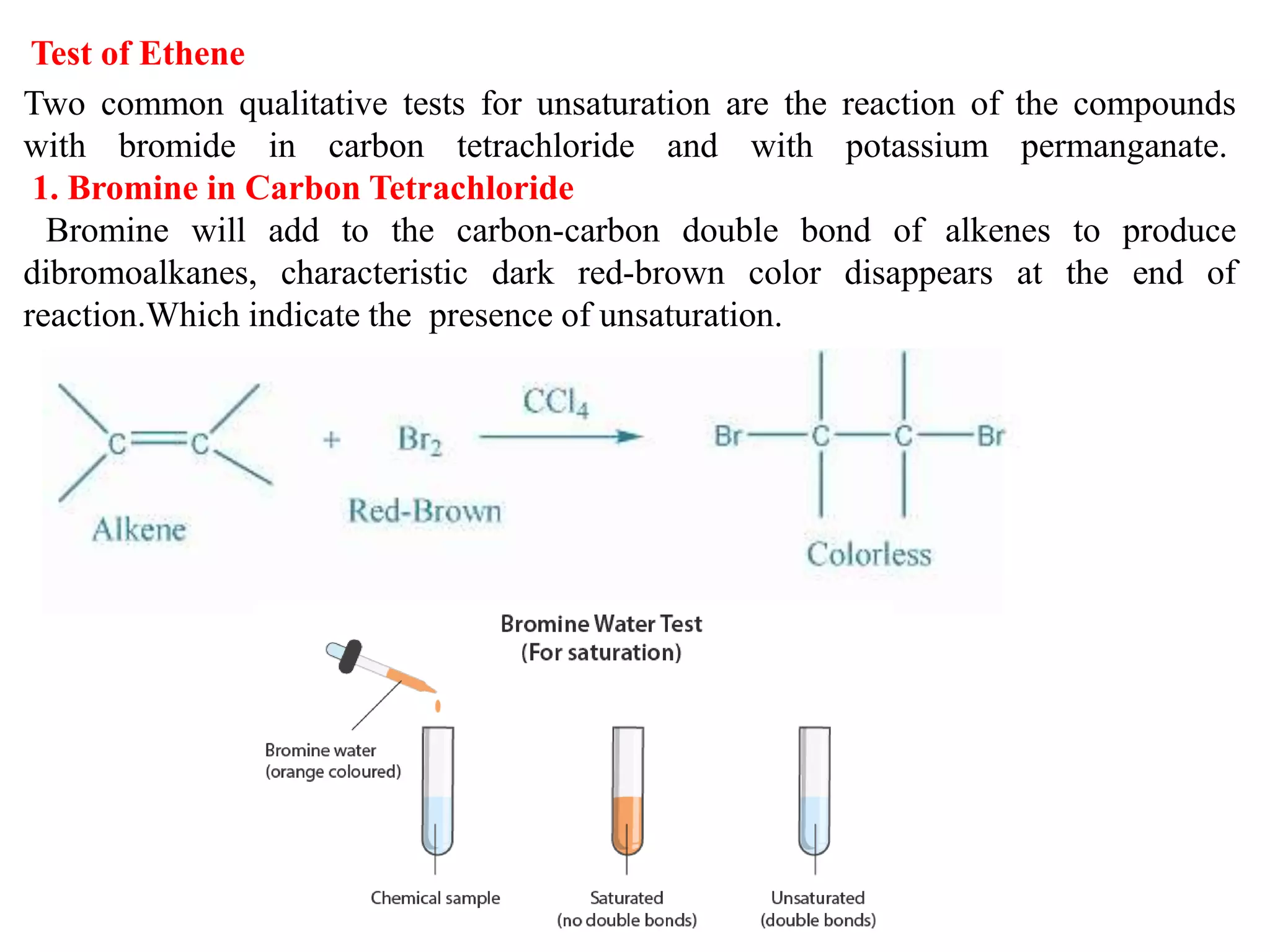
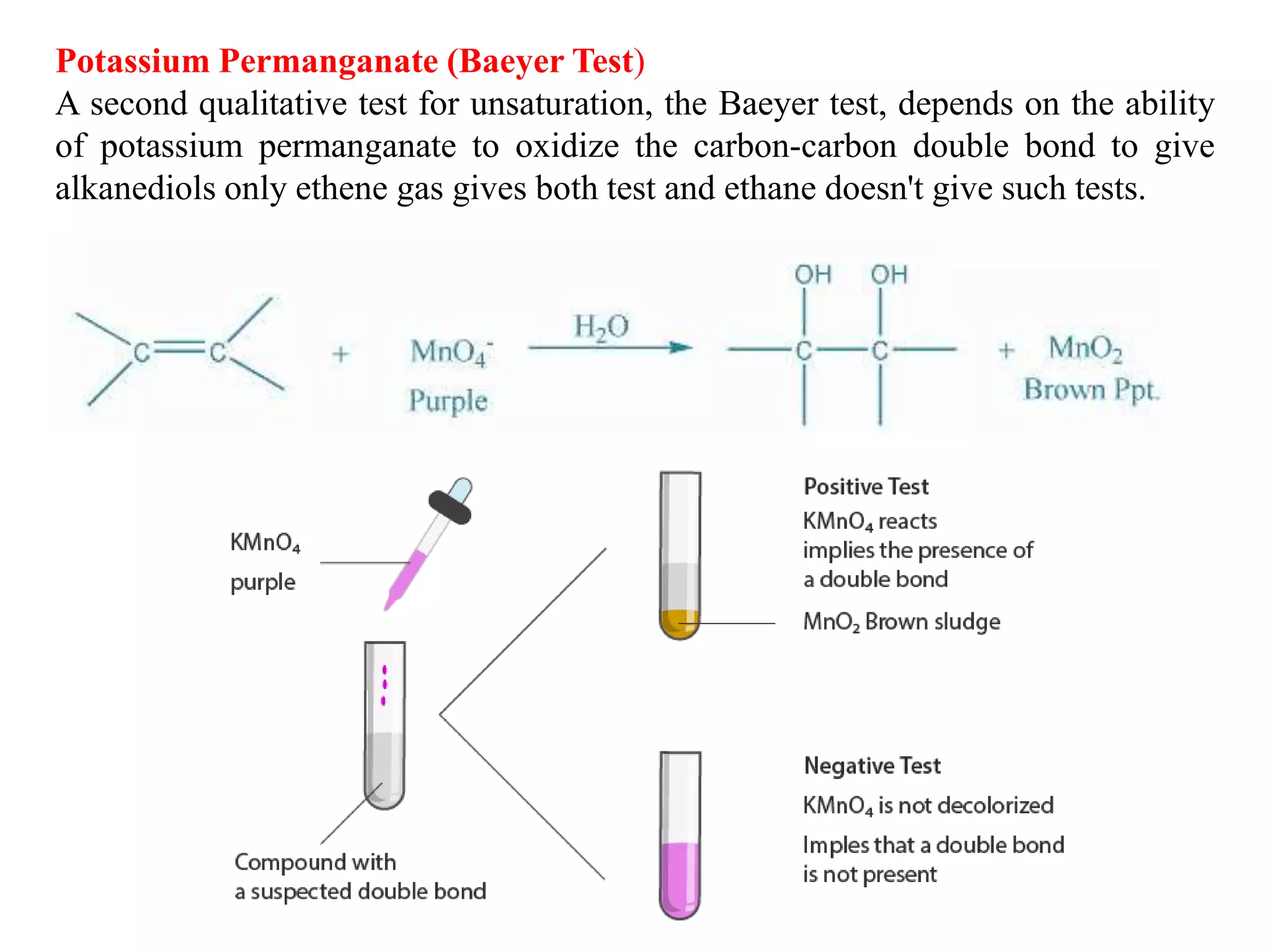
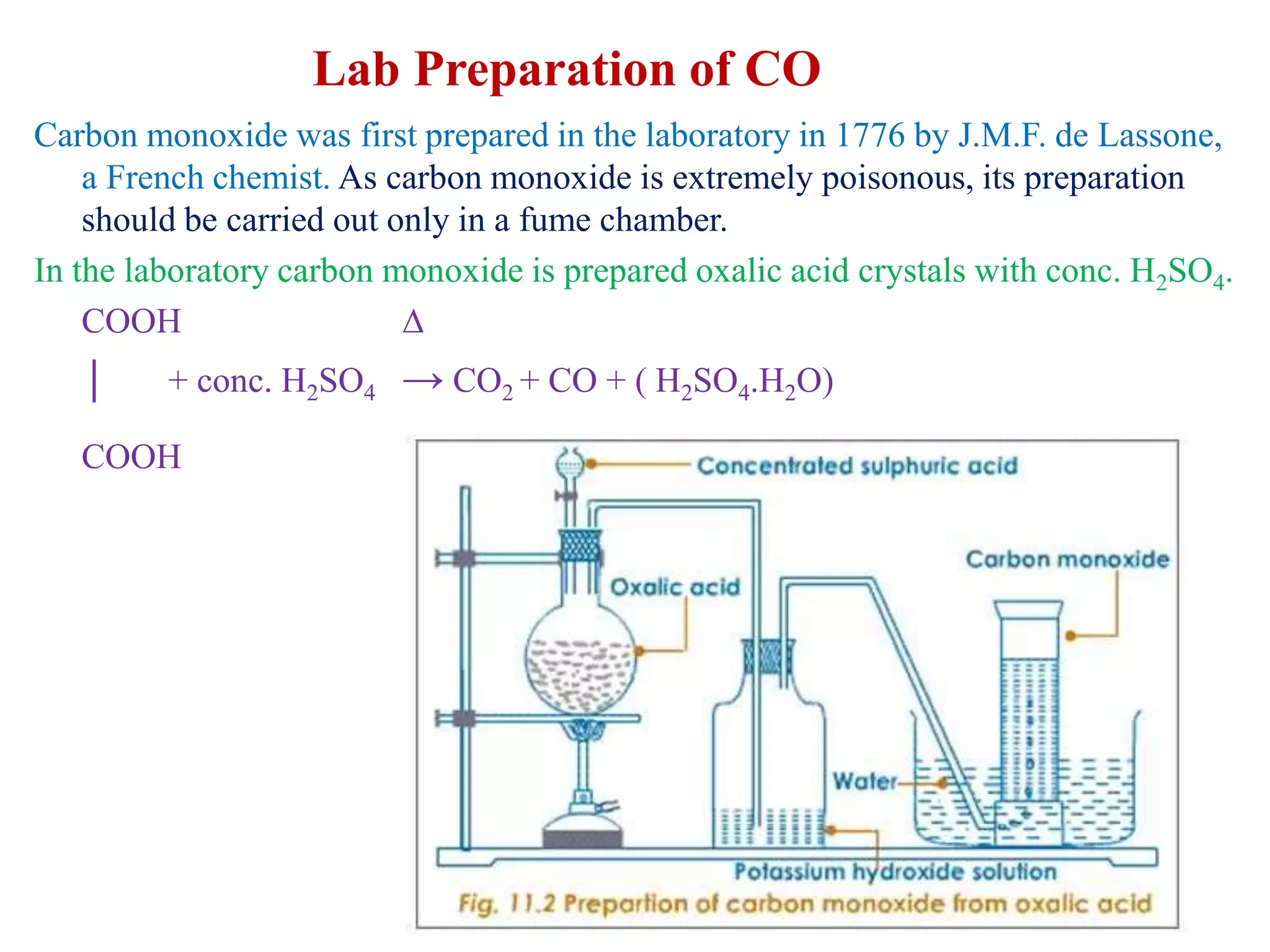
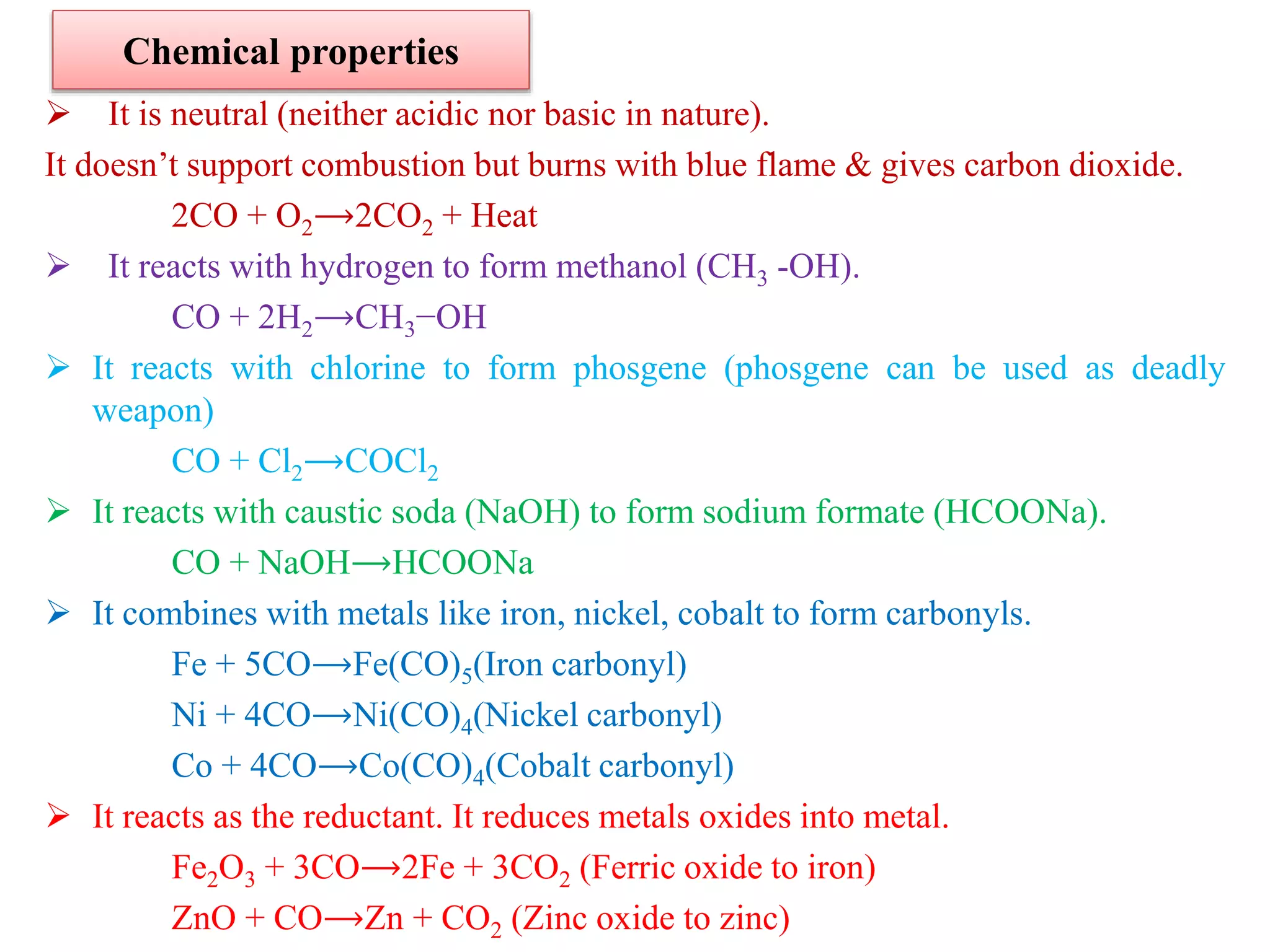
This document provides instructions for preparing several common laboratory gases and chemicals. It describes how to produce carbon monoxide by heating oxalic acid crystals with concentrated sulfuric acid. Hydrogen iodide is prepared by dropping water onto a mixture of red phosphorus and iodine. Hydrogen gas is produced through the reaction of zinc and dilute sulfuric acid. Hydrogen sulfide gas can be prepared using iron sulfide and hydrochloric acid in Kipp's apparatus. Sulfur dioxide is formed from copper turnings and concentrated sulfuric acid. Ozone is generated by passing oxygen through an electric discharge. Physical and chemical properties are also outlined for each substance.



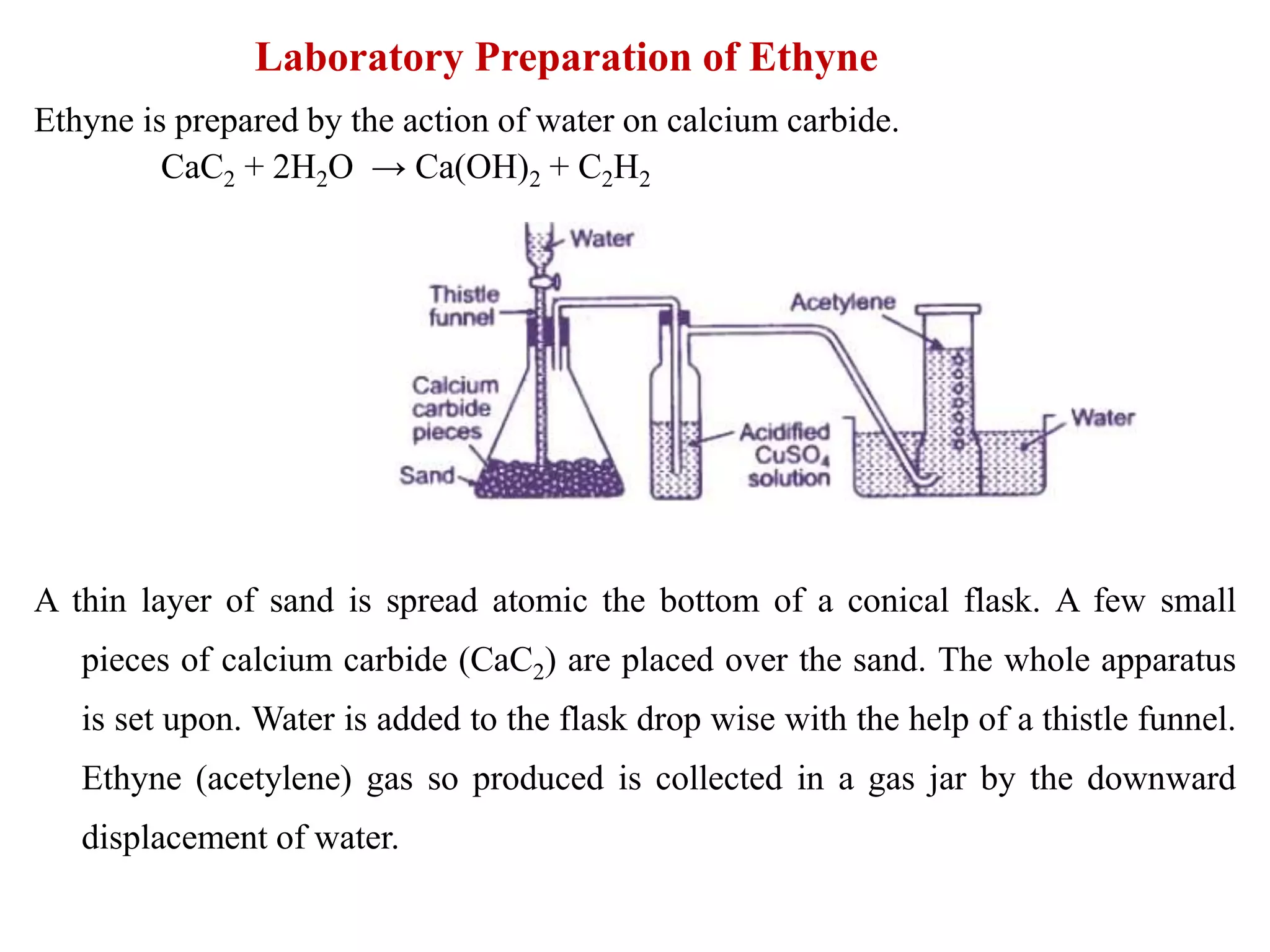
![Laboratory preparation of HI
In the laboratory, HI is prepared by dropping water on a mixture of red phosphorus
and iodine. A mixture of iodine and red phosphorus is taken in the flask and water
is dropped from the funnel. HI with some vapour of iodine is evolved.
P4 + 6I2 → 4PI3
PI3 + 3H2O → 3HI + H3PO3 ] * 4
P4 + 6I2 + 12H2O → 12HI + 4H3PO3
HI cannot be prepared in the same way as HCl by using conc. H2SO4 because HI
being stronger reducing agent, reduces the oxidizing acid (i.e.conc. H2SO4) to
SO2.
2HI + H2SO4 → SO2 + 2H2O + I2](https://image.slidesharecdn.com/labpreparationxi-200508130344/75/Lab-preparation-XI-7-2048.jpg)