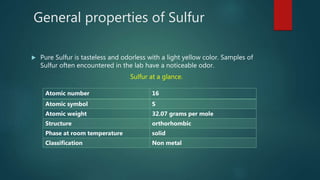
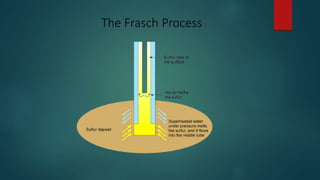
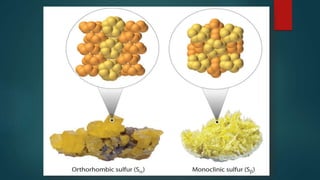
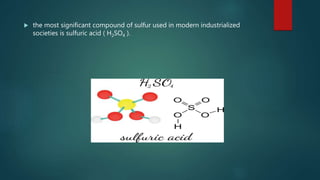
Sulfur exists in many forms including elemental sulfur and in compounds. It is a nonmetal that is extracted using the Frasch process. Sulfur has several allotropes depending on temperature and exists as S-8 rings at room temperature. Important sulfur compounds include sulfur dioxide, sulfur trioxide, hydrogen sulfide, and sulfuric acid. Sulfuric acid is the most widely produced sulfur compound and is used mainly in fertilizer production.