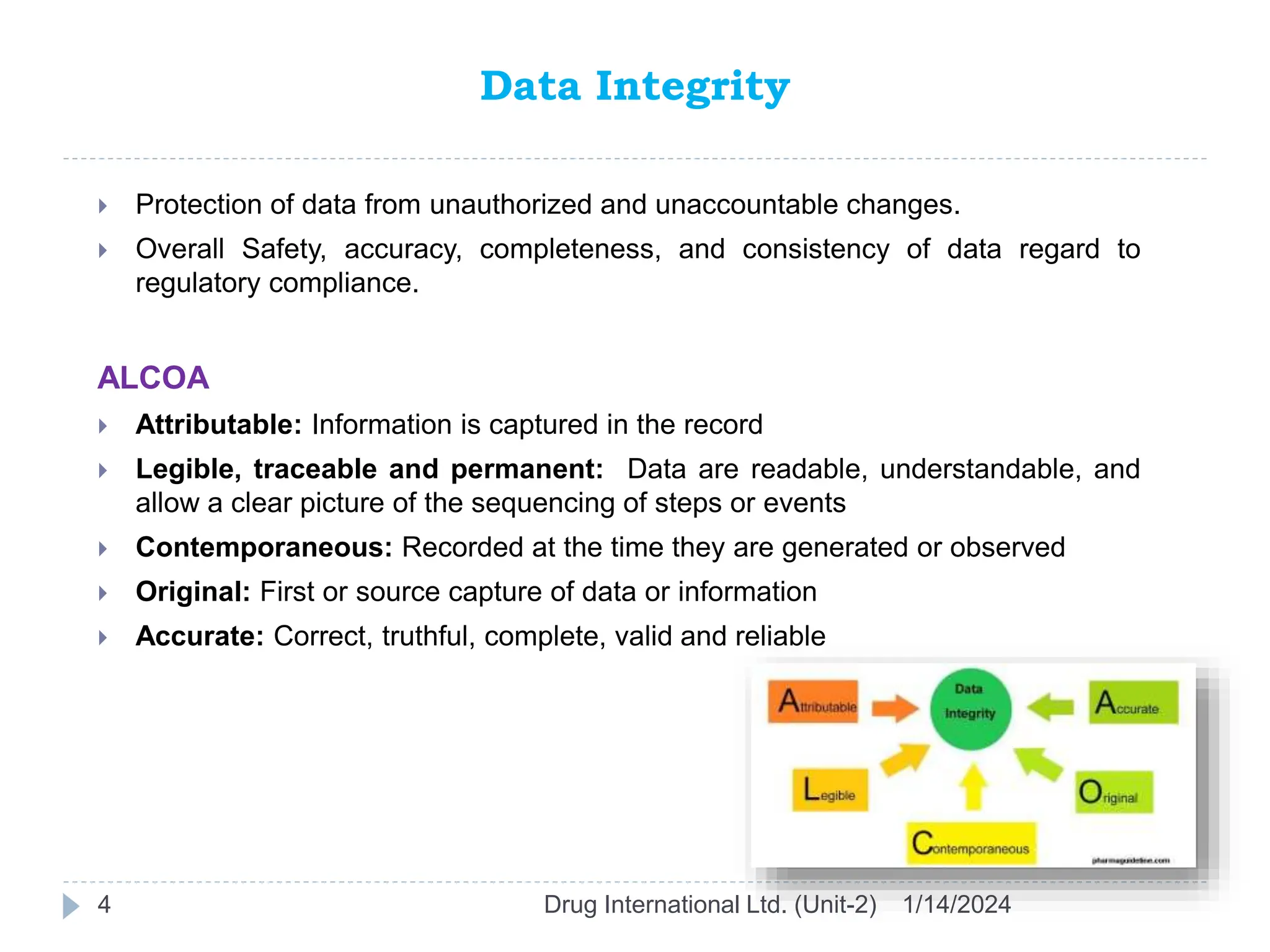
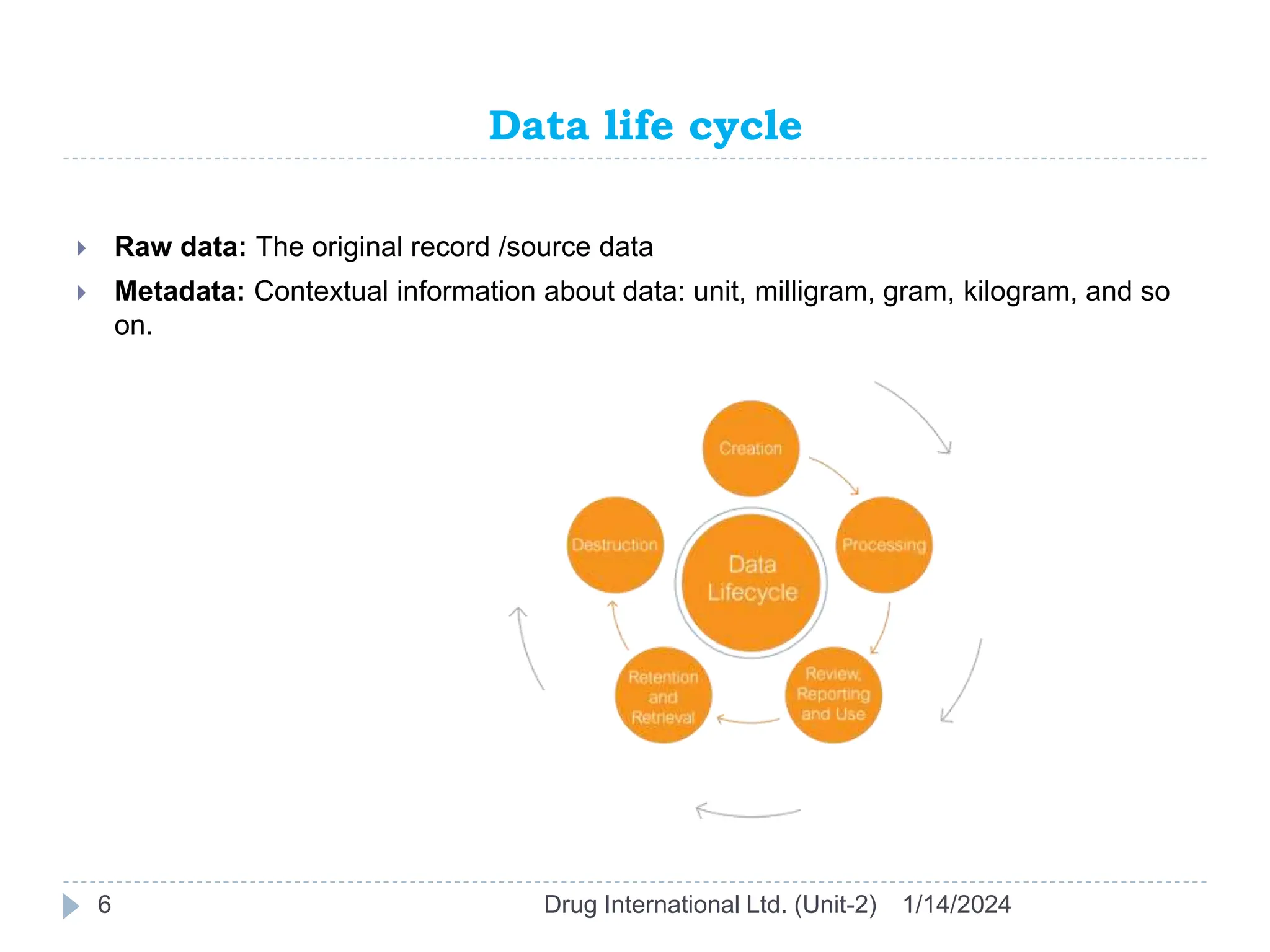
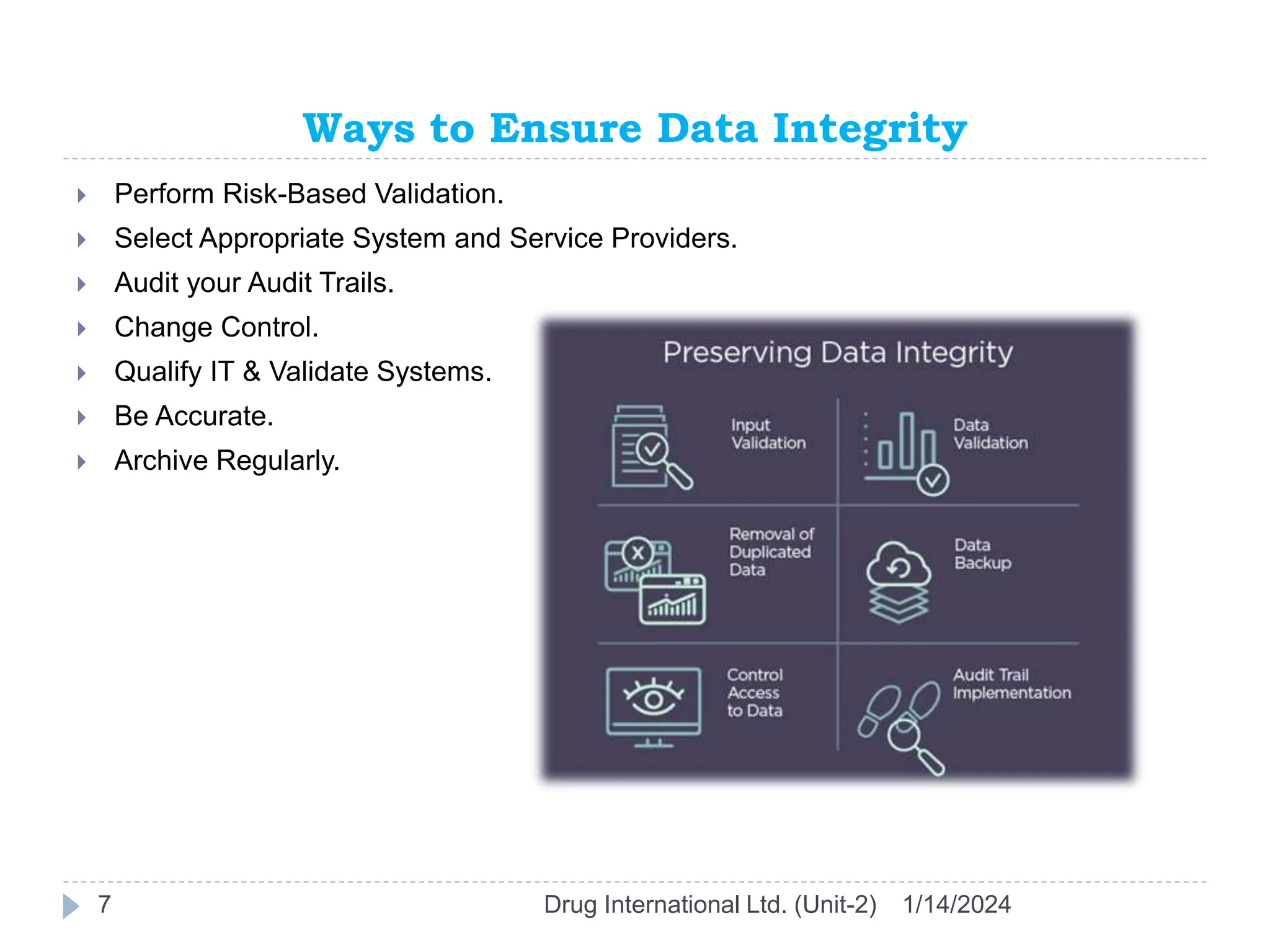
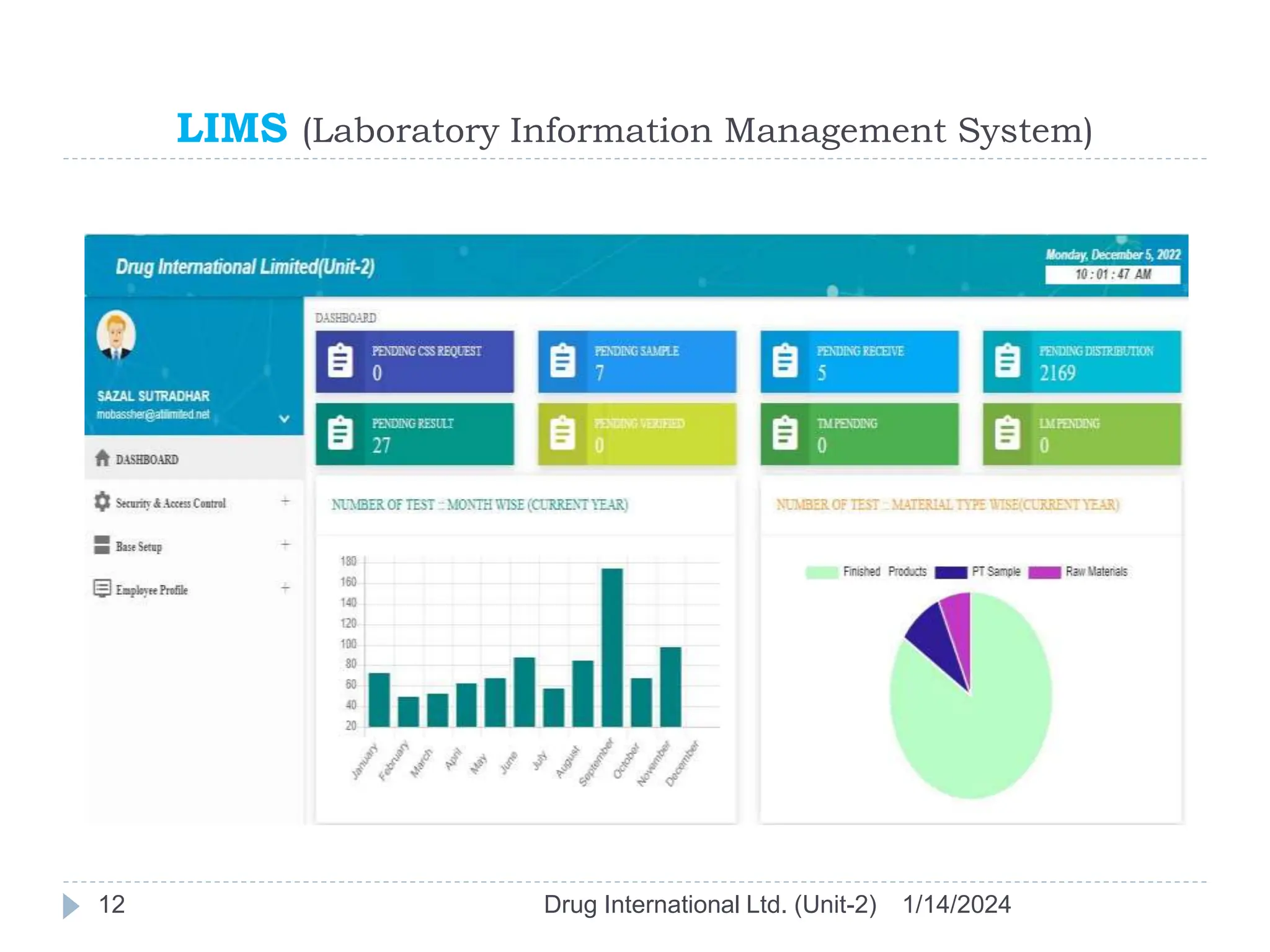
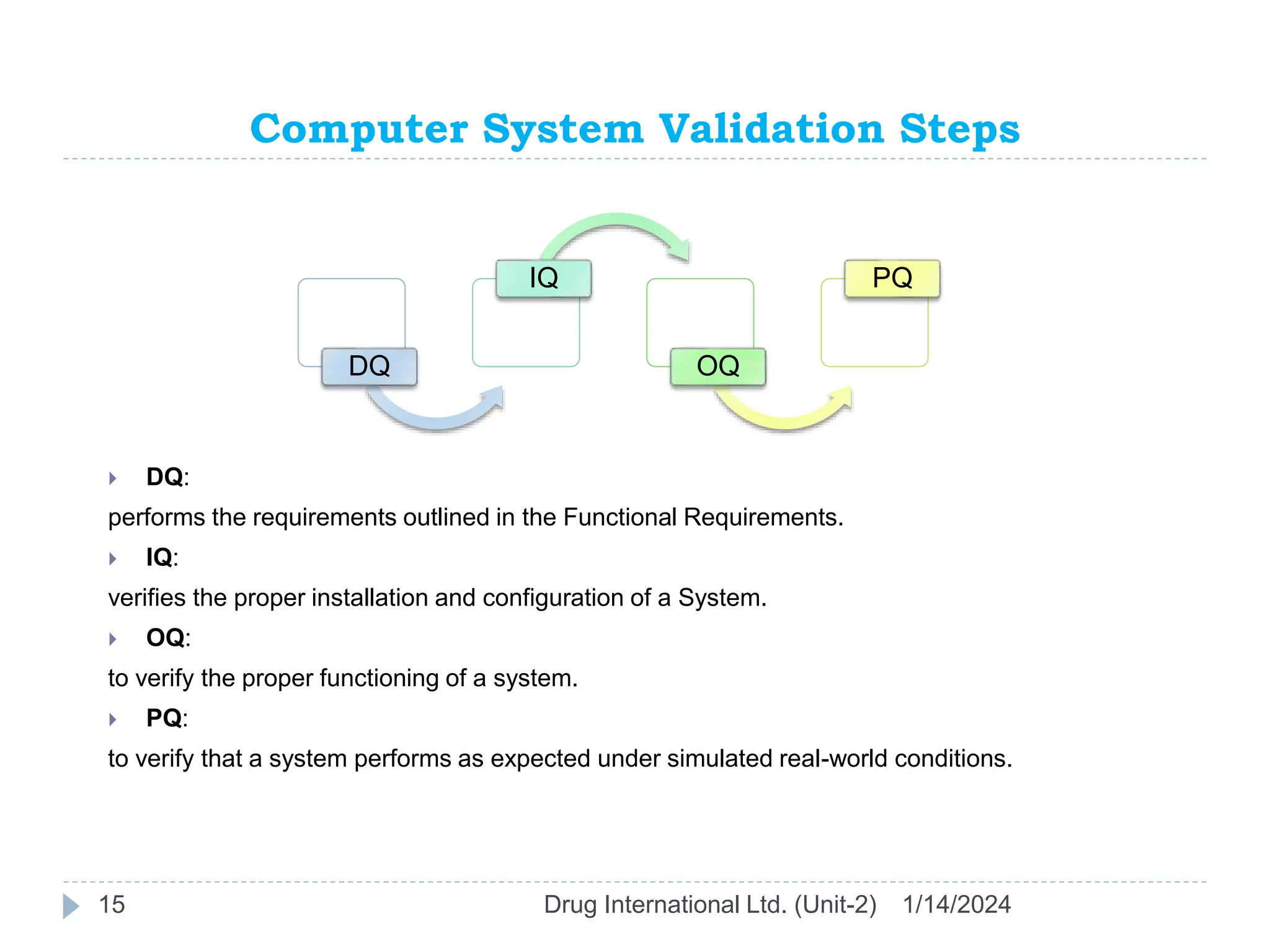
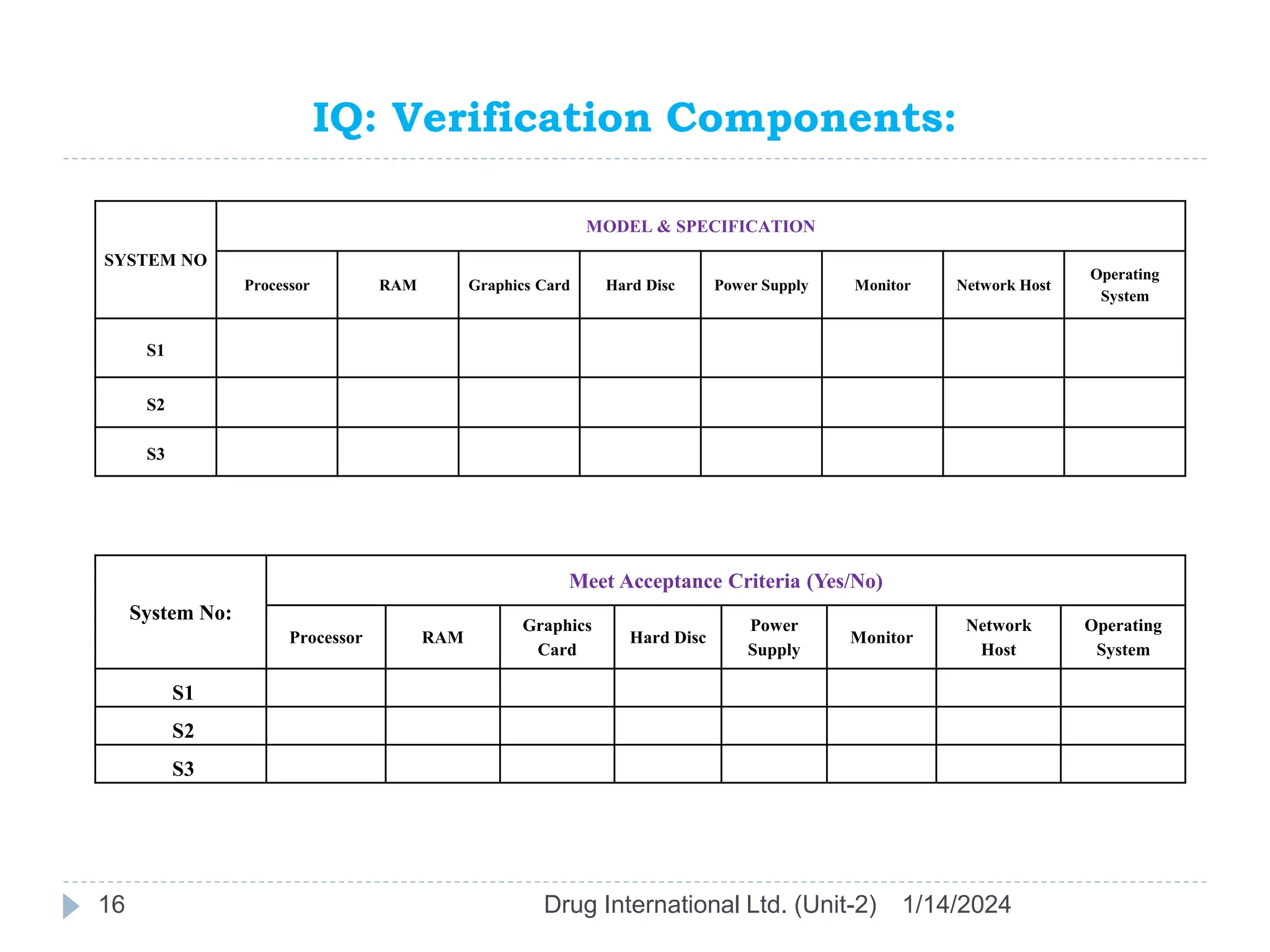
The document outlines the GAMP approach to data integrity, electronic records, signatures, and the operation of GxP computerized systems crucial for the pharmaceutical industry. It emphasizes the importance of data integrity, electronic data, and proper validation processes to ensure compliance and quality in pharmaceutical practices. Key responsibilities and methods for maintaining data integrity and validation of computerized systems are also discussed, along with definitions of GXP regulations.