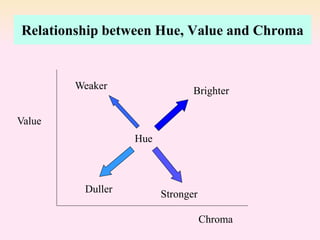
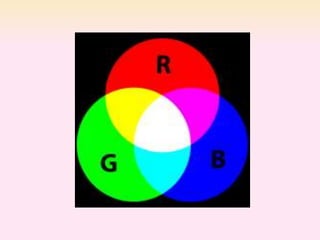
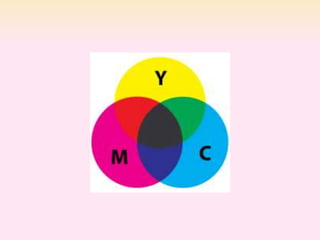
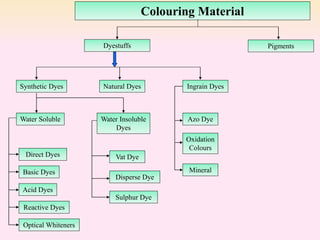
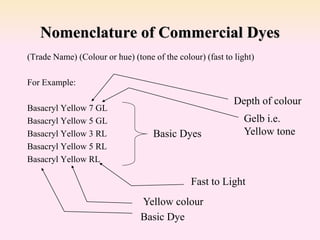
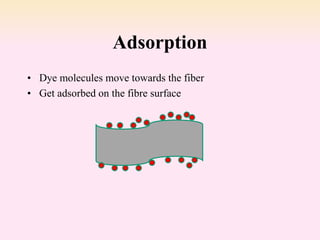
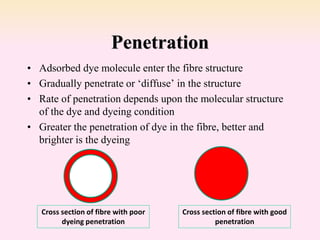
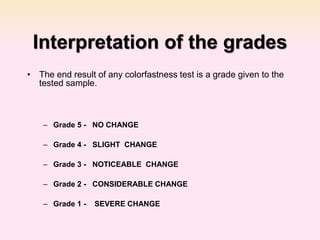
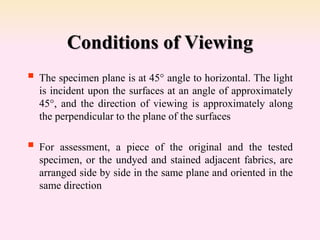
Dyeing textiles makes them more attractive and functional. There are three main factors that define color: hue (the color name), value (lightness or darkness), and chroma (brightness or dullness). Dyes are applied to textiles through dyeing or printing processes. Various dye types include reactive, acid, basic, disperse and pigment dyes. Proper dye selection and dyeing conditions such as temperature, time and chemicals are required to achieve optimal color fastness properties. Color fastness testing evaluates how color withstands effects like washing, light exposure, rubbing and perspiration.