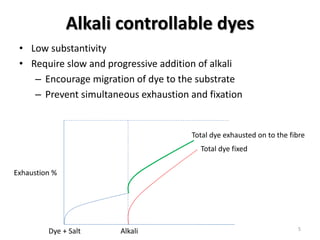
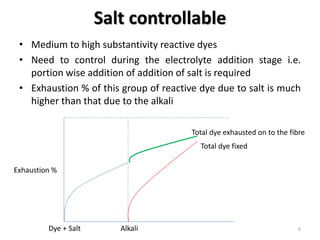
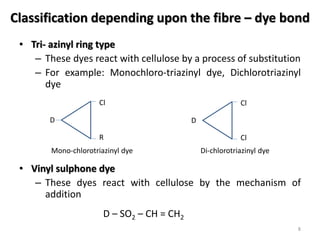
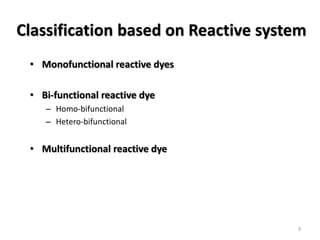
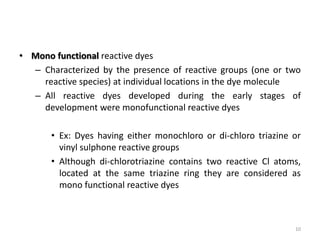
Reactive dyes readily dissolve in water and form covalent bonds with cellulose fibers, providing good washing fastness. They are classified based on their reactivity - alkaline controllable dyes require gradual alkali addition, salt controllable dyes require portion-wise salt addition, and temperature controllable dyes react above boiling temperatures. Bifunctional reactive dyes contain two reactive groups, allowing for high exhaustion and fixation during dyeing of cotton which involves dyeing with salt, forming covalent bonds with alkali, and post-treatment processes like soaping and washing.