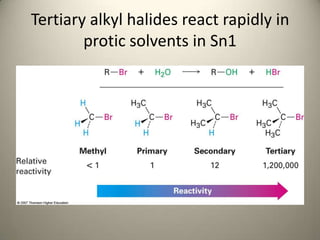
The document discusses two reactions involving seven alkyl halides: 1) treatment with sodium iodide in acetone, which favors an Sn2 reaction, and 2) treatment with ethanolic silver nitrate solution, which favors an Sn1 reaction. For the Sn2 reaction, a non-polar solvent like acetone is used, favoring the reaction pathway. For the Sn1 reaction, a polar protic solvent like ethanol is used, promoting ionization of the alkyl halide. The rate of each reaction is observed by noting the time of appearance of any precipitate formed.




![Sodium Iodide in Acetone
favors the Sn2 reaction
nucleophile
Electrophile Rate = k[ROTs][OAc]
Leaving Group](https://image.slidesharecdn.com/7alkylhalidesnew-121120125209-phpapp01/85/7-alkyl-halides-new-6-320.jpg)

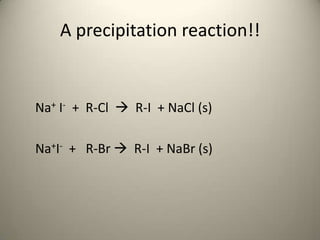

![Rate-determining step is formation of
carbocation
rate = k[RX]](https://image.slidesharecdn.com/7alkylhalidesnew-121120125209-phpapp01/85/7-alkyl-halides-new-10-320.jpg)