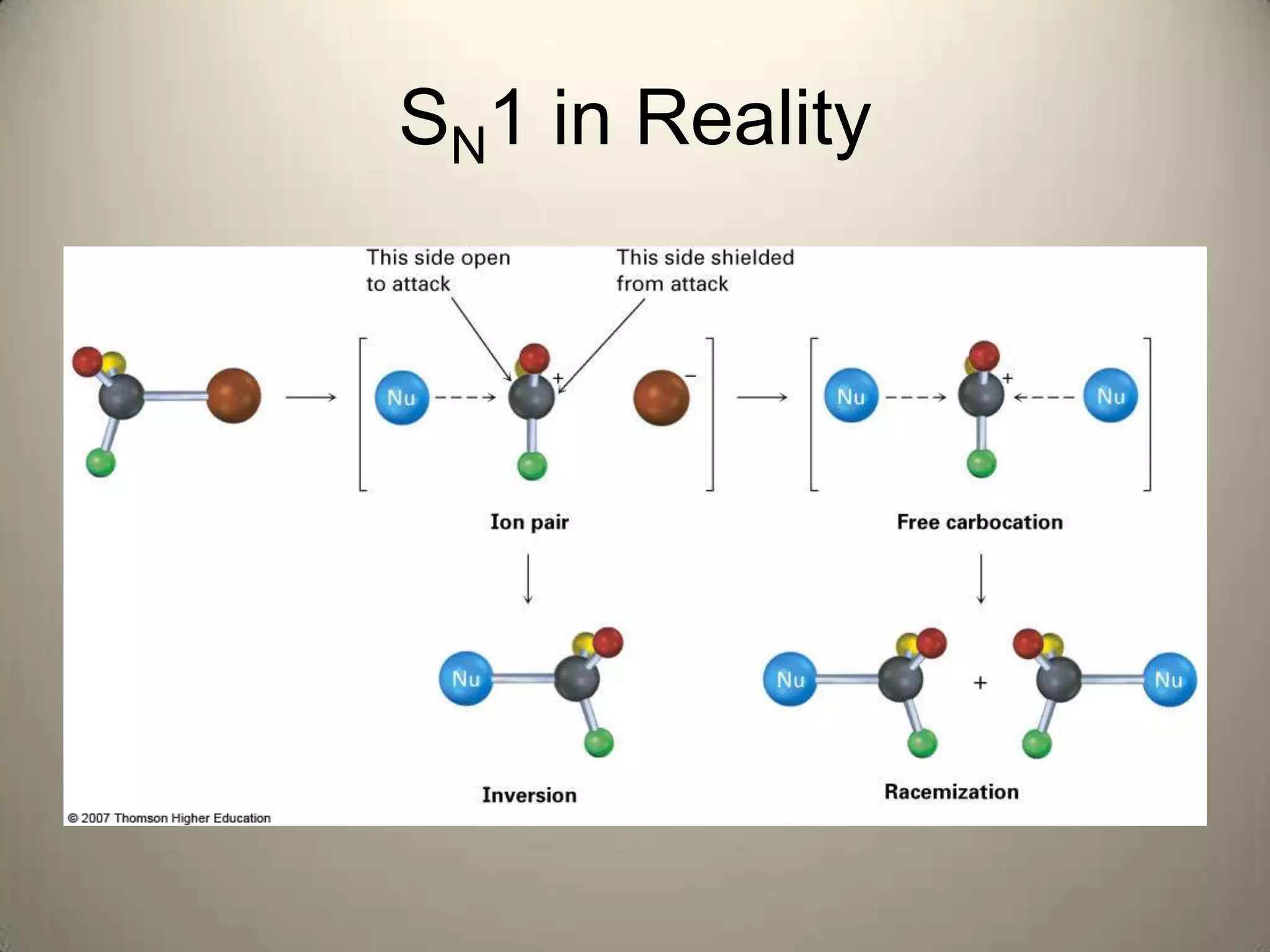
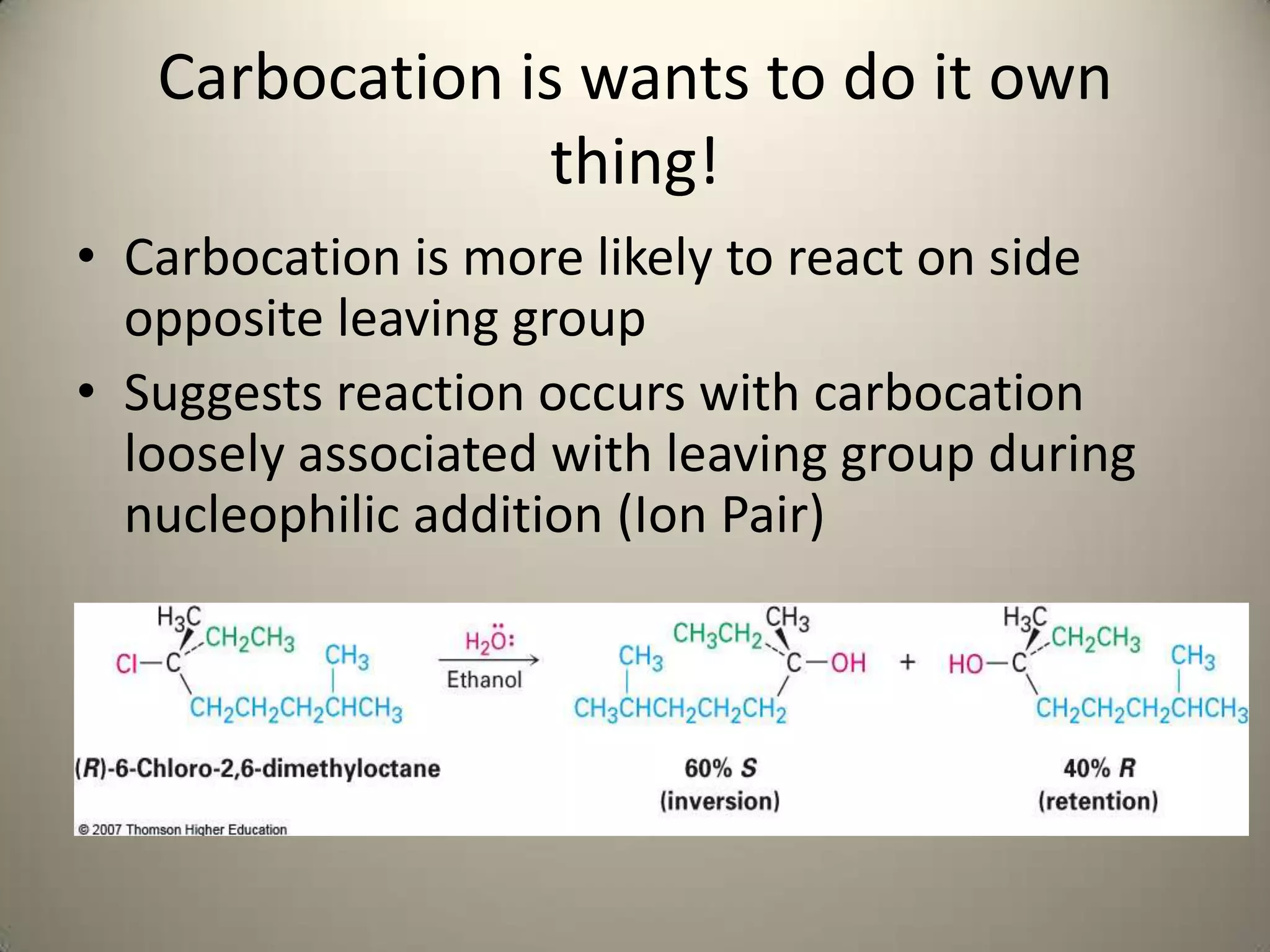
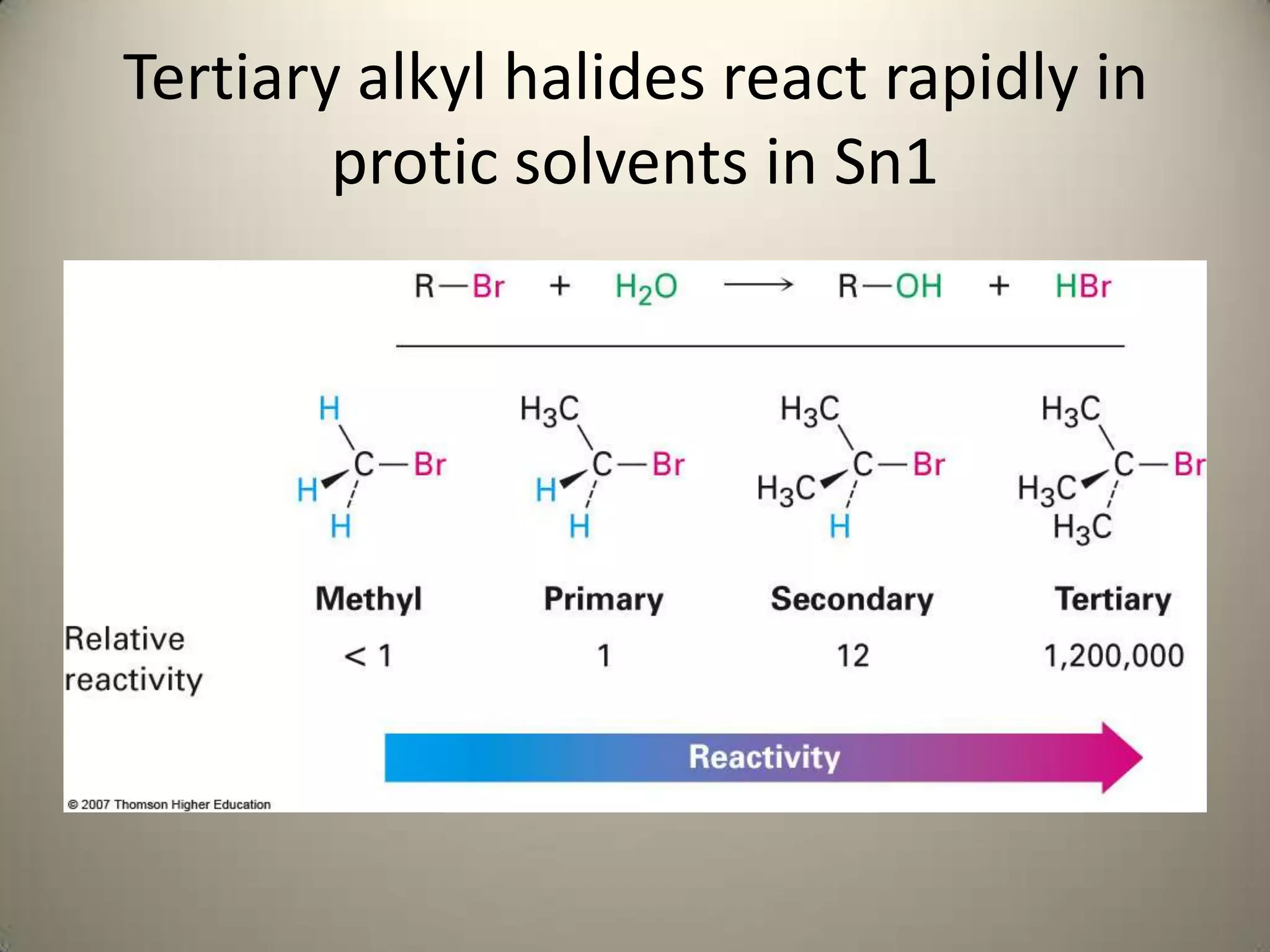
The document outlines a laboratory procedure for treating seven alkyl halides with sodium iodide in acetone and ethanolic silver nitrate to observe SN2 and SN1 reactions. It highlights that sodium iodide favors the SN2 reaction due to its nonpolar solvent properties, while ethanolic silver nitrate promotes SN1 reactions by facilitating carbocation formation. The document includes specific instructions for conducting microscale experiments to observe these reactions and analyze factors influencing reactivity.



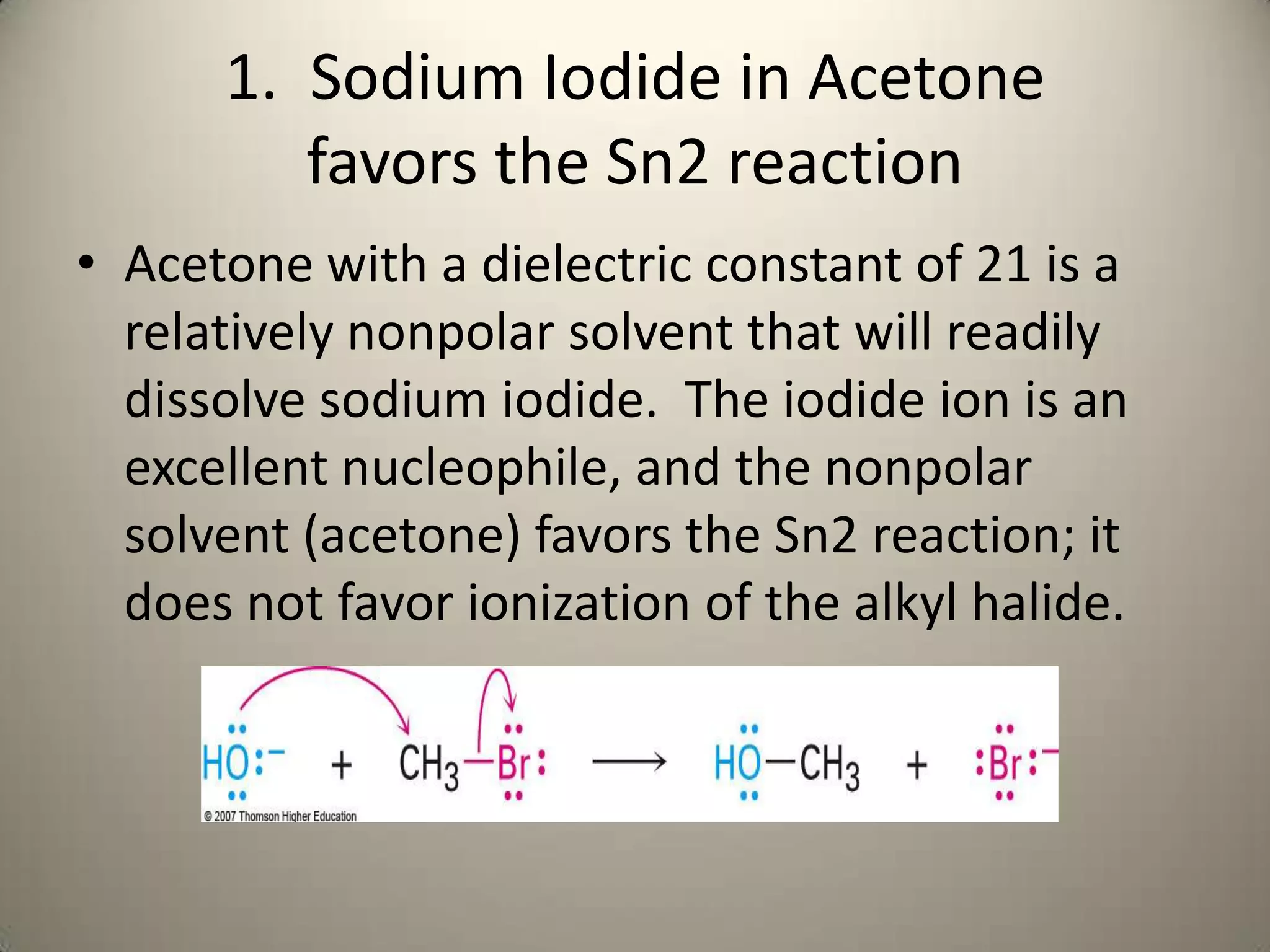
![Sodium Iodide in Acetone
favors the Sn2 reaction
nucleophile
Electrophile Rate = k[ROTs][OAc]
Leaving Group](https://image.slidesharecdn.com/presentation17alkylhalidesnew2-121204125804-phpapp02/75/Presentation17-alkyl-halides-new-2-5-2048.jpg)



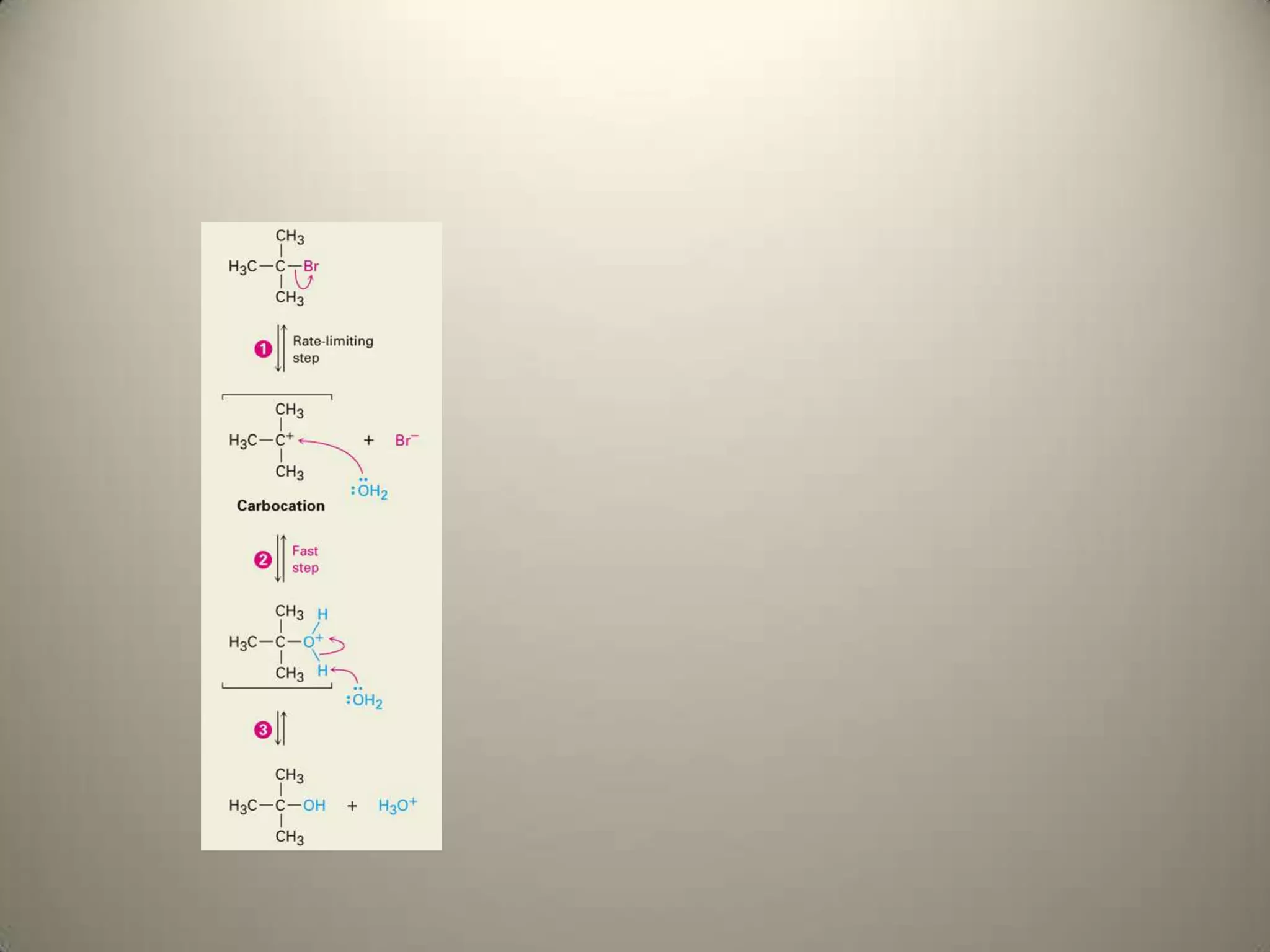
![Rate-determining step is formation of
carbocation
rate = k[RX]](https://image.slidesharecdn.com/presentation17alkylhalidesnew2-121204125804-phpapp02/75/Presentation17-alkyl-halides-new-2-10-2048.jpg)