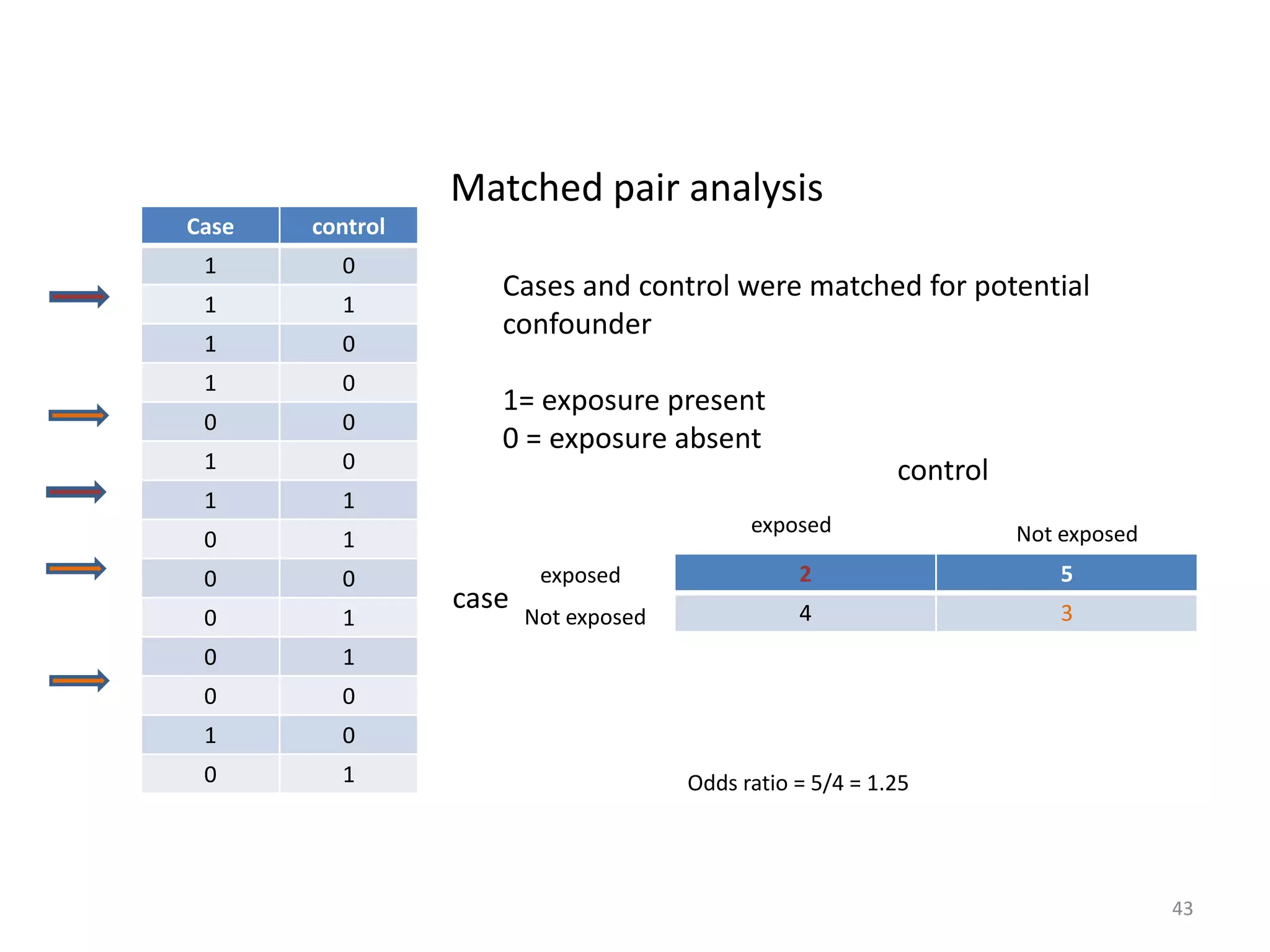
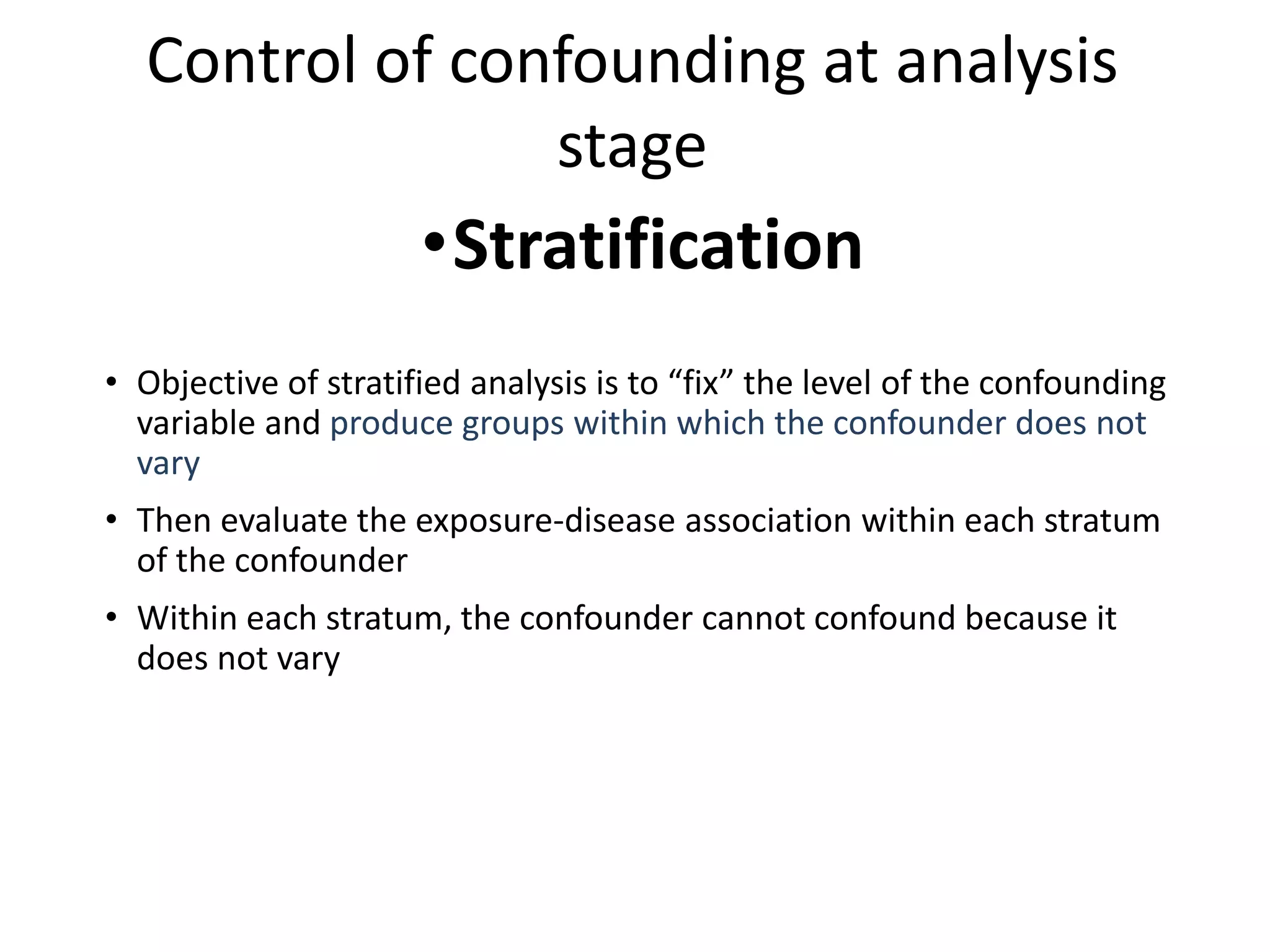
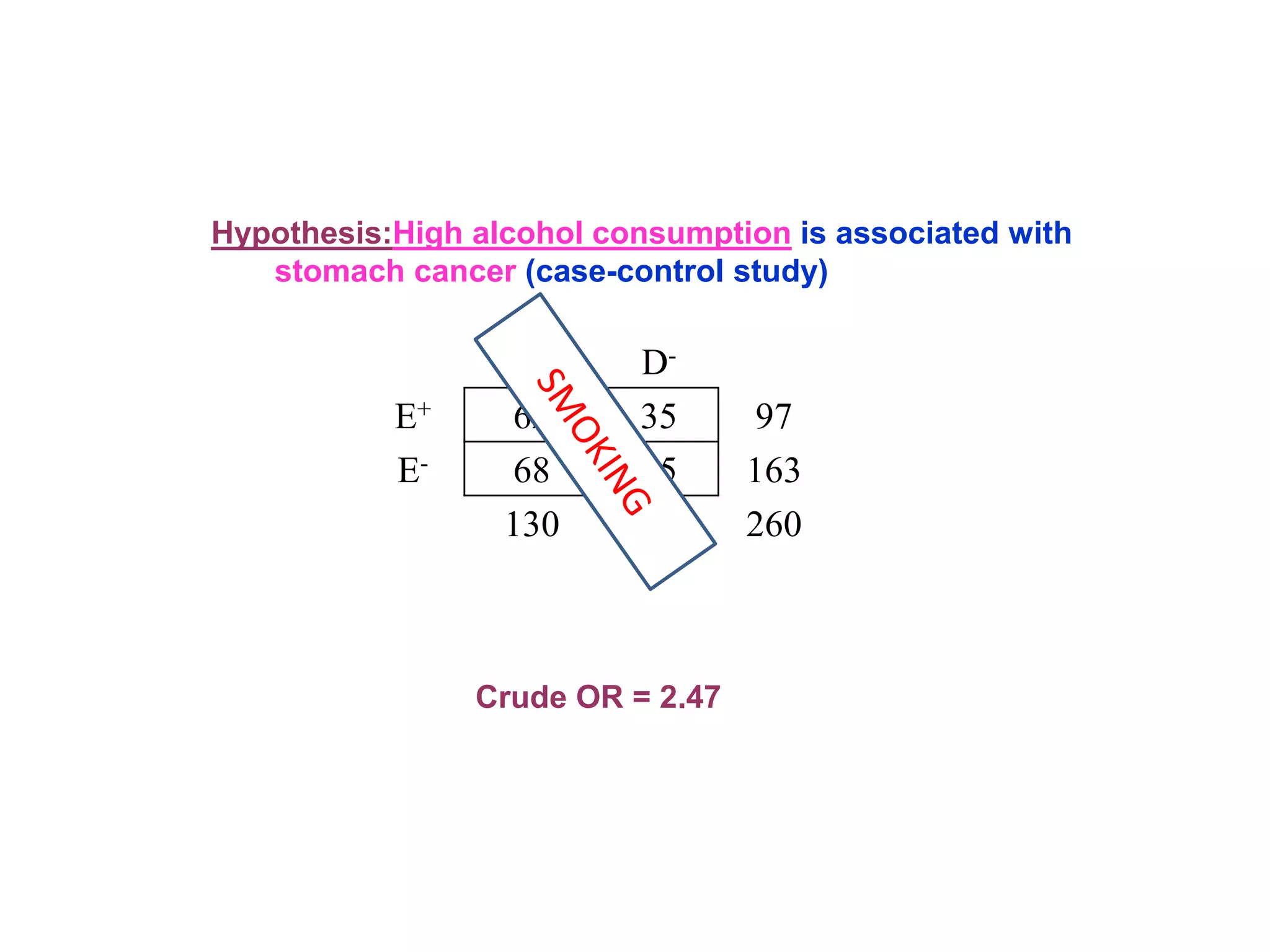
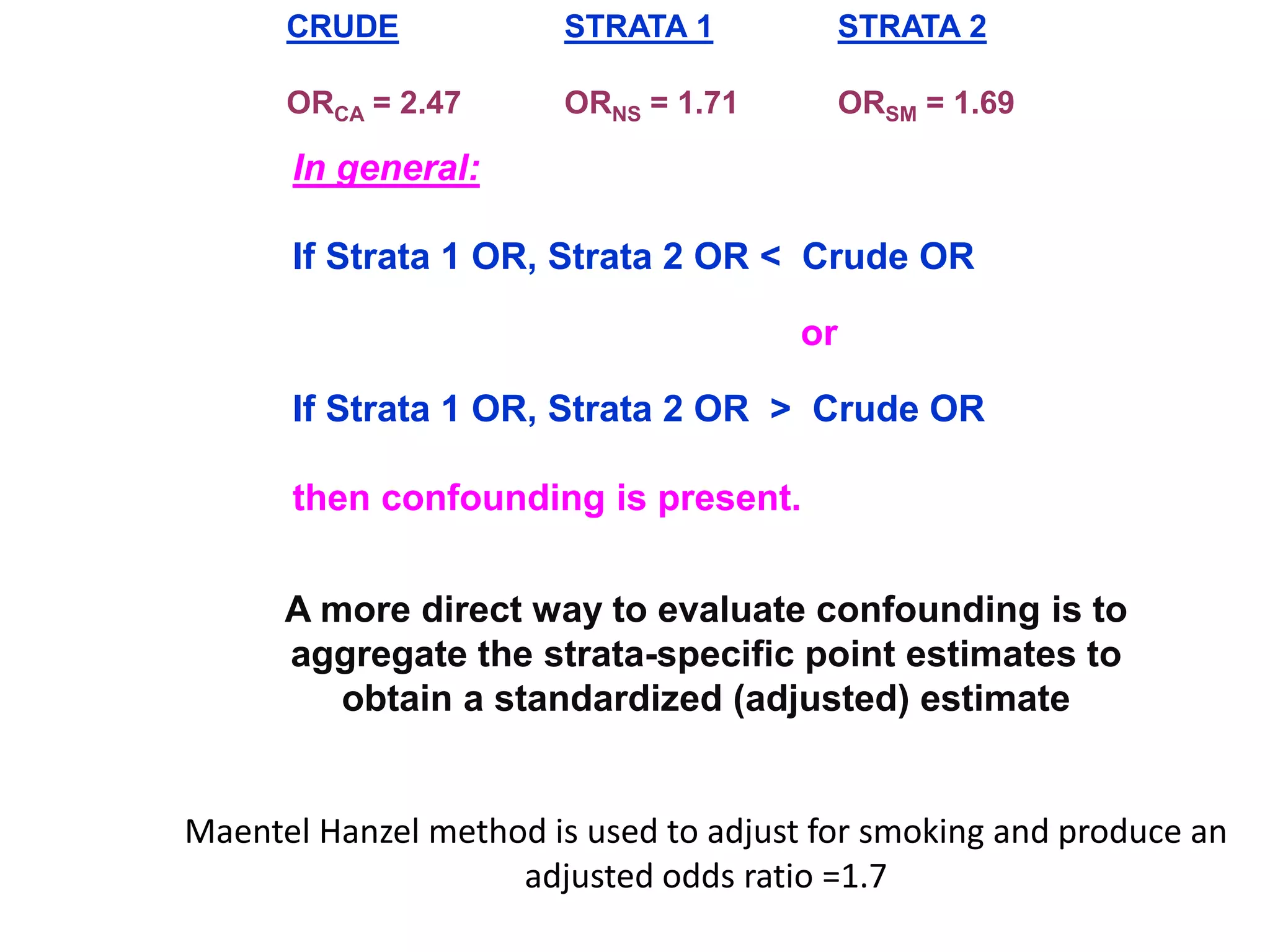
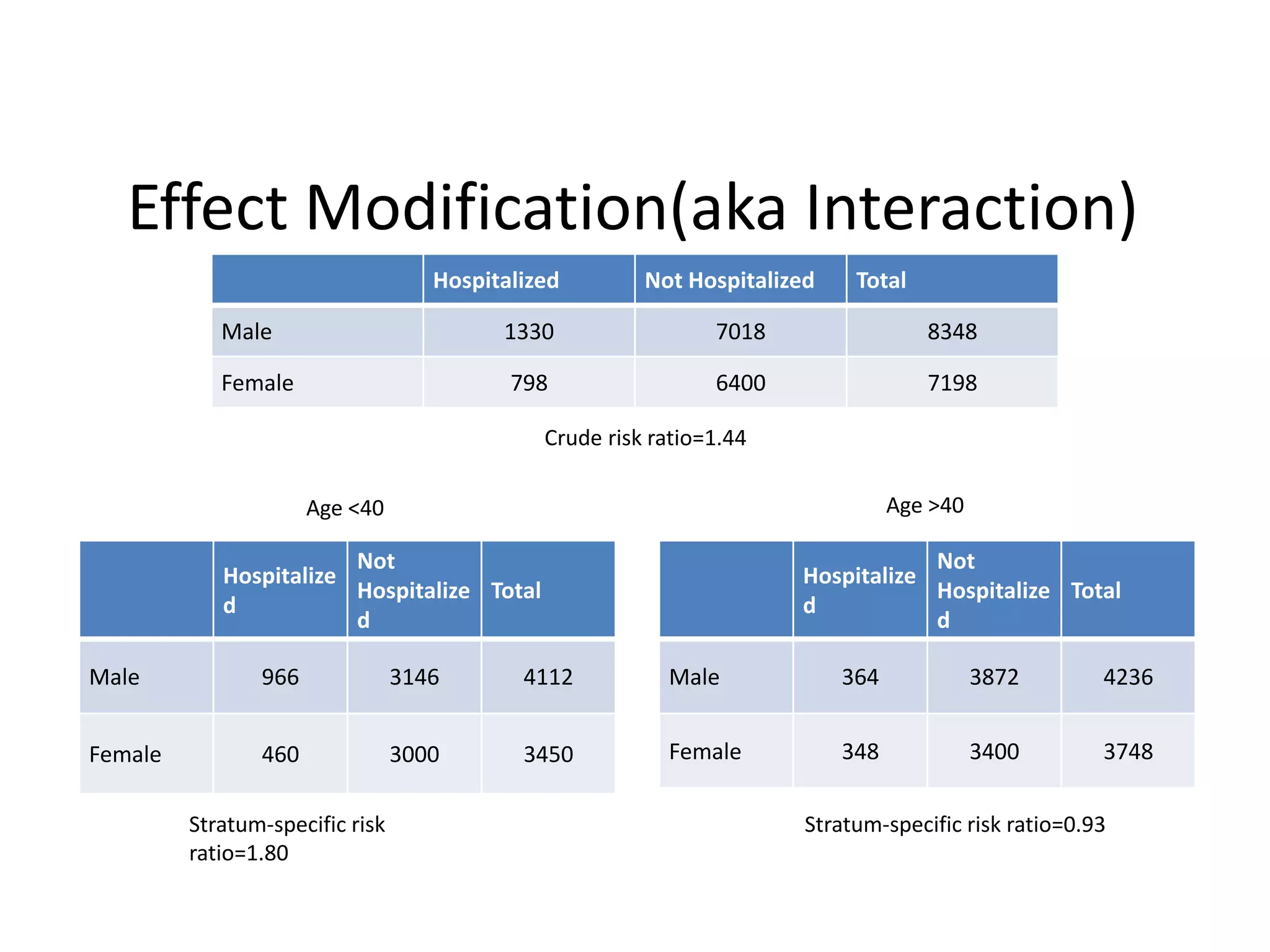
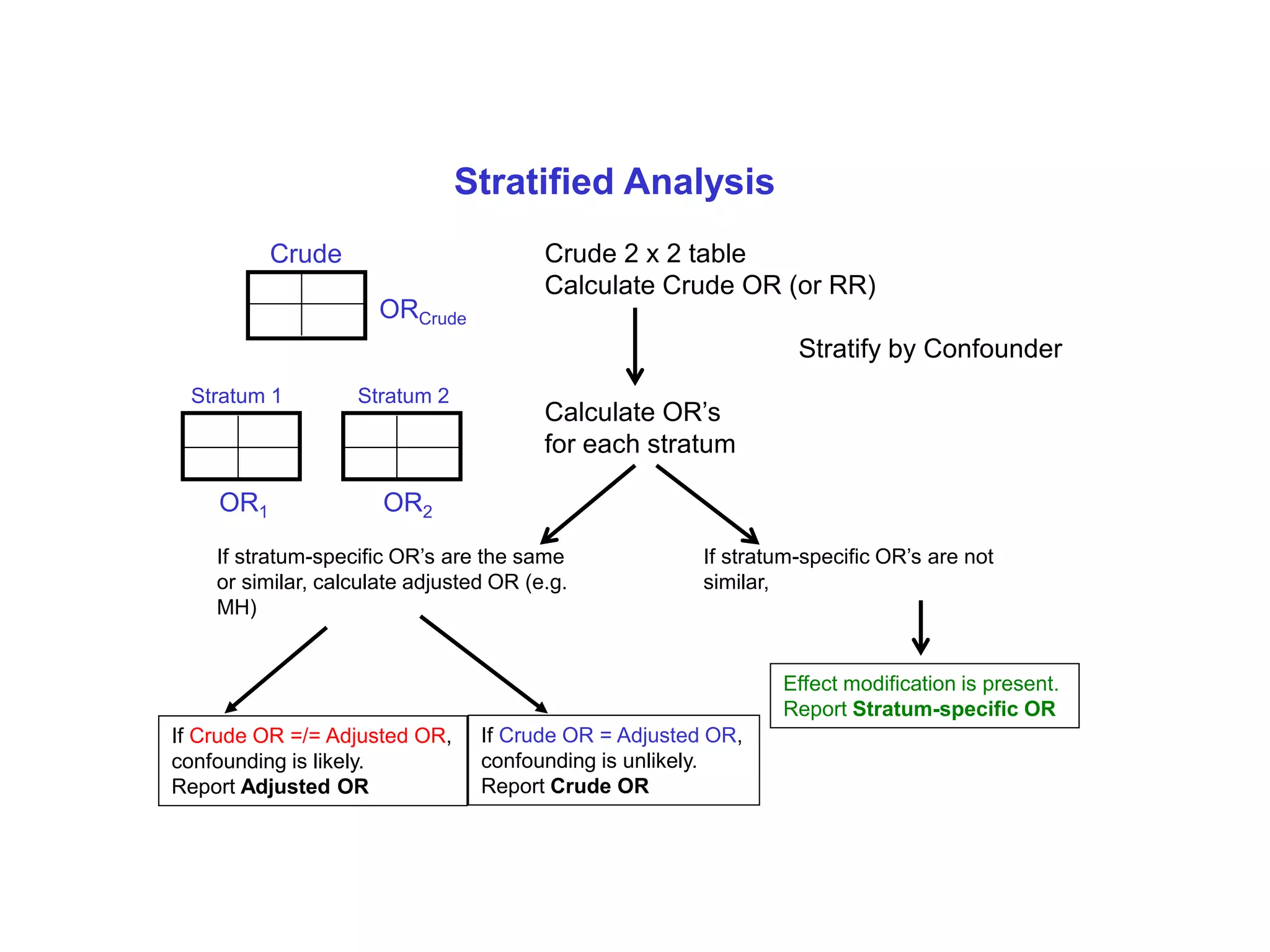
This document discusses various types of bias and confounding that can occur in epidemiological studies, including:
1) Selection bias, information bias, and confounding can all introduce systematic error and invalidate study results. Selection bias occurs when individuals have different probabilities of being included based on exposure or outcome. Information bias is misclassification of exposure or outcome.
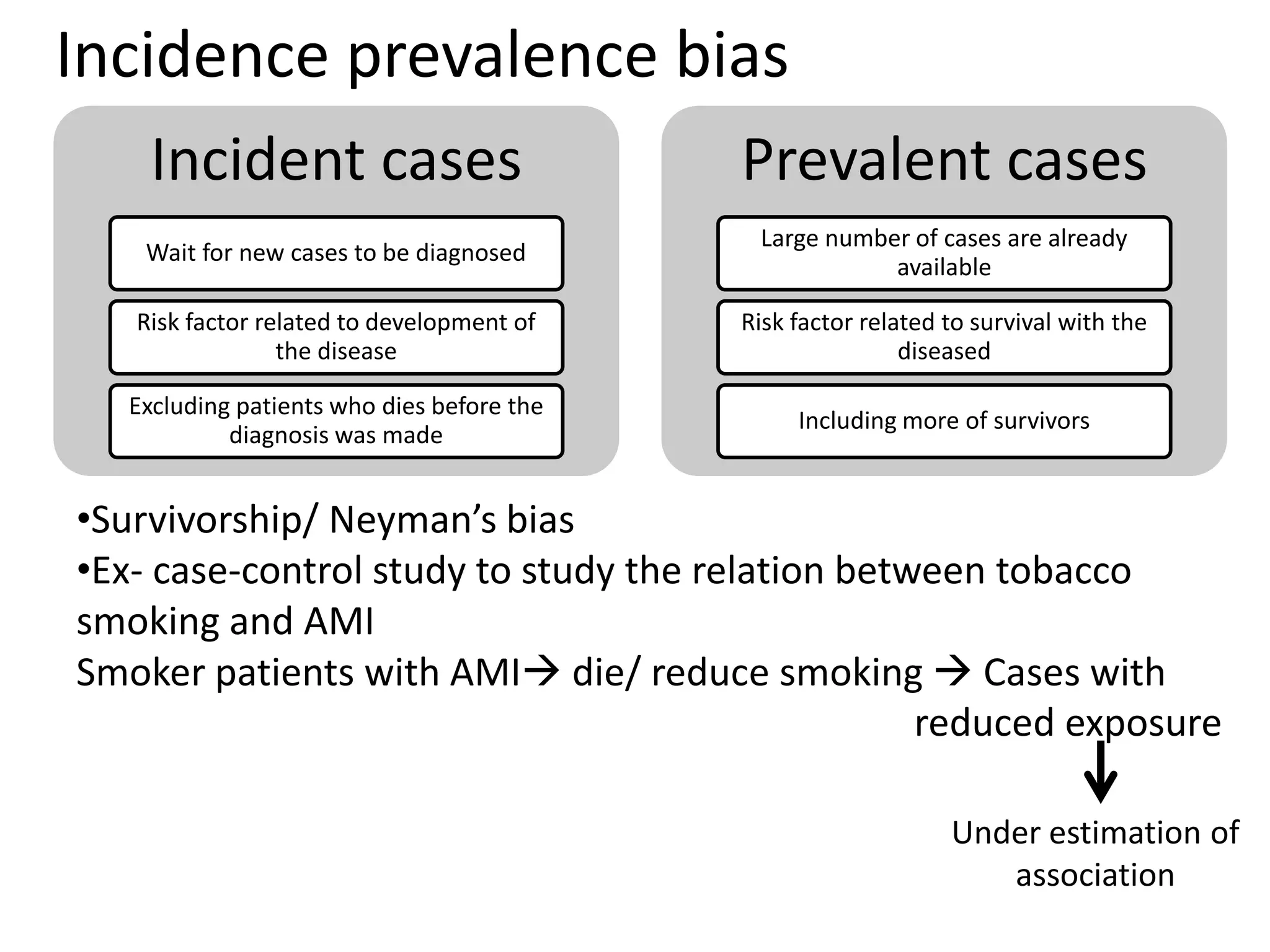
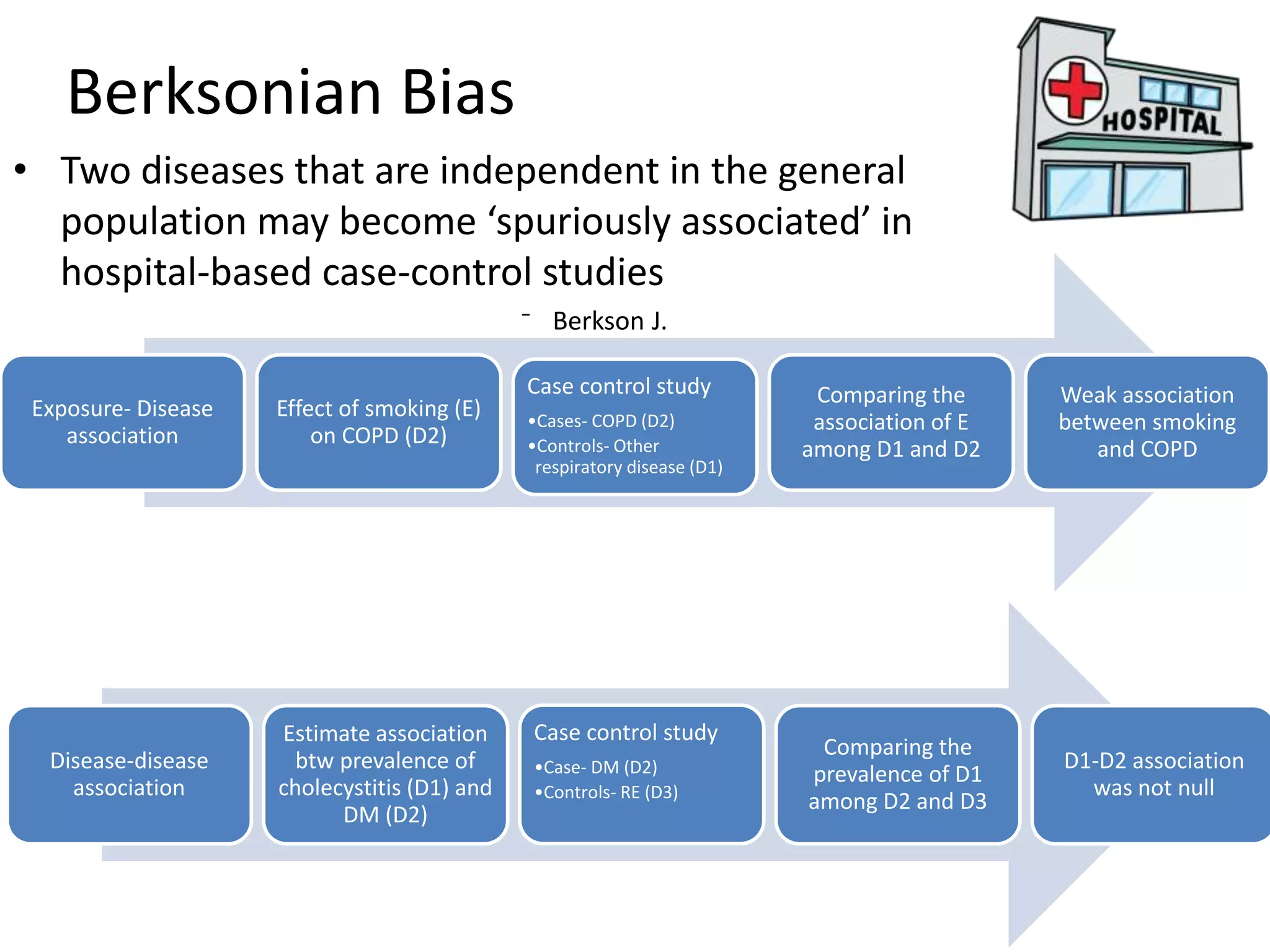
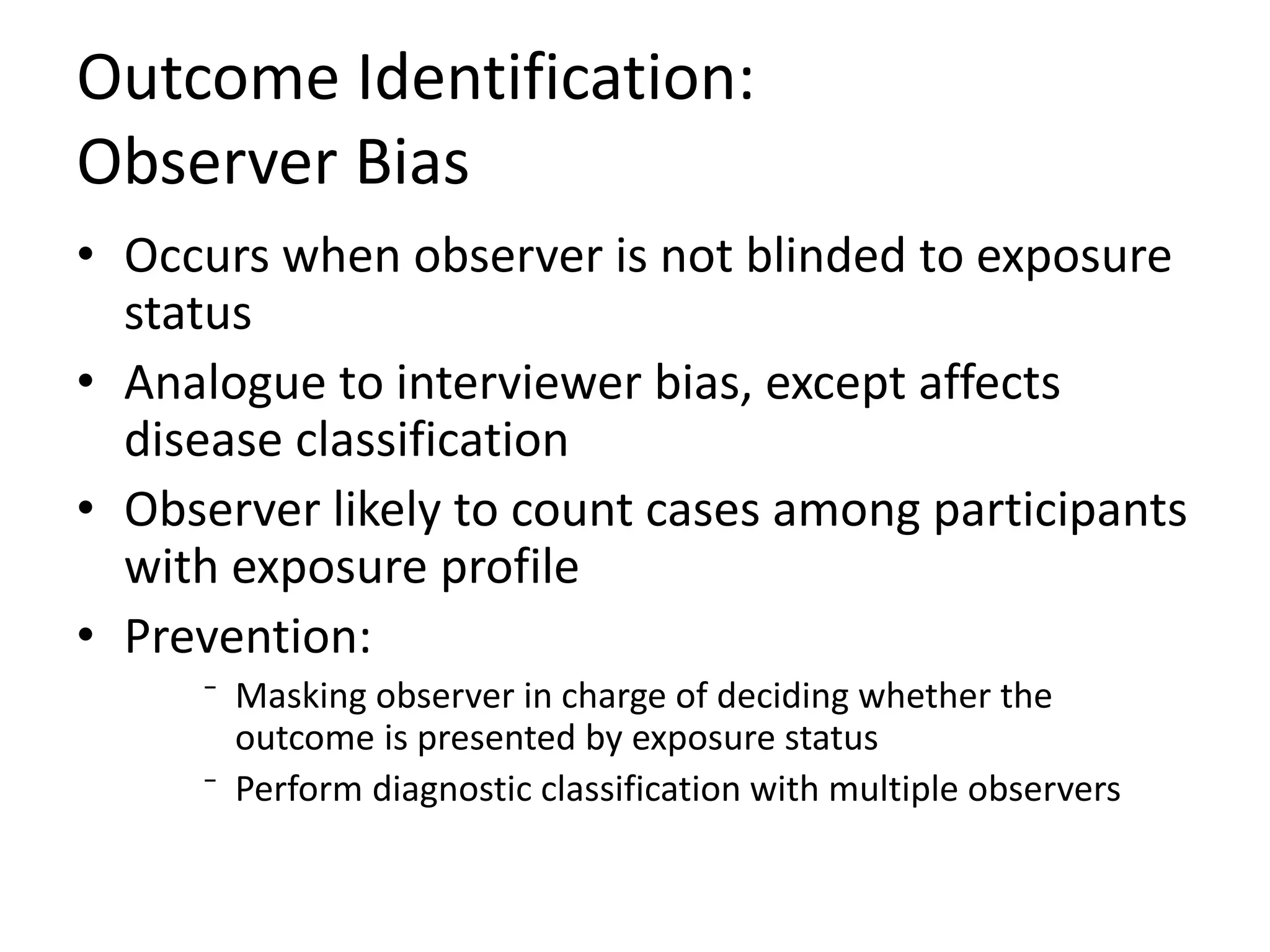
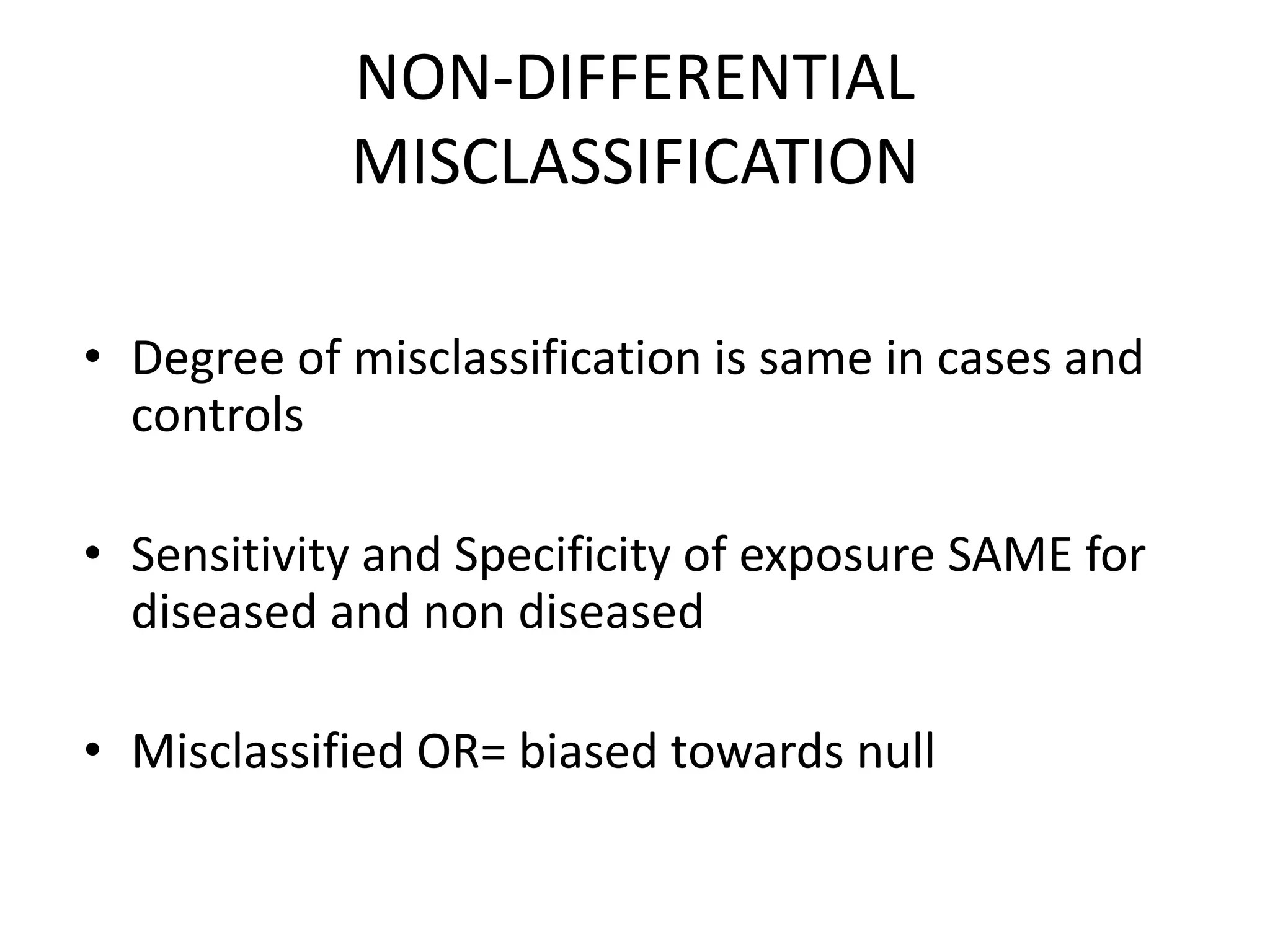
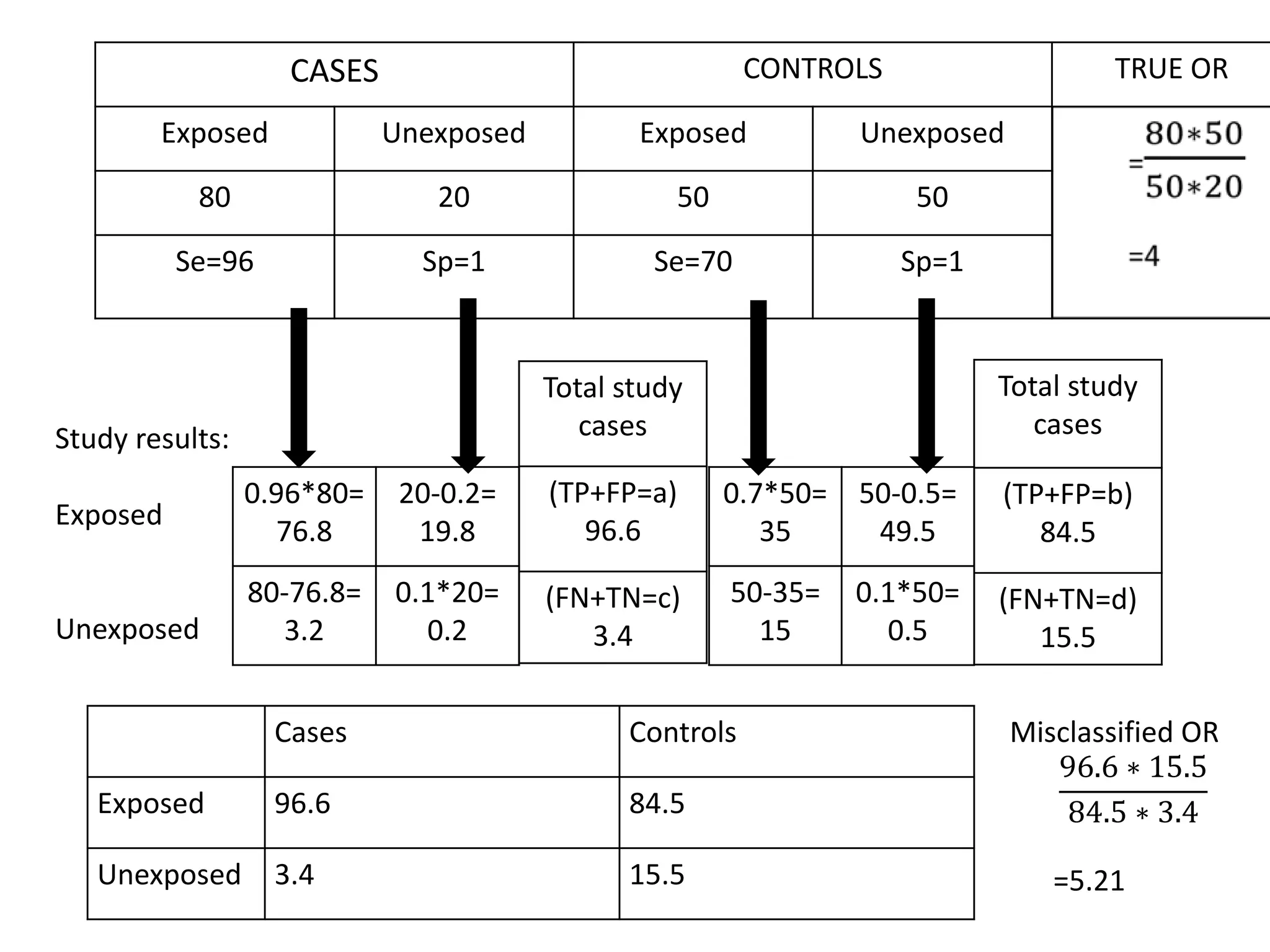
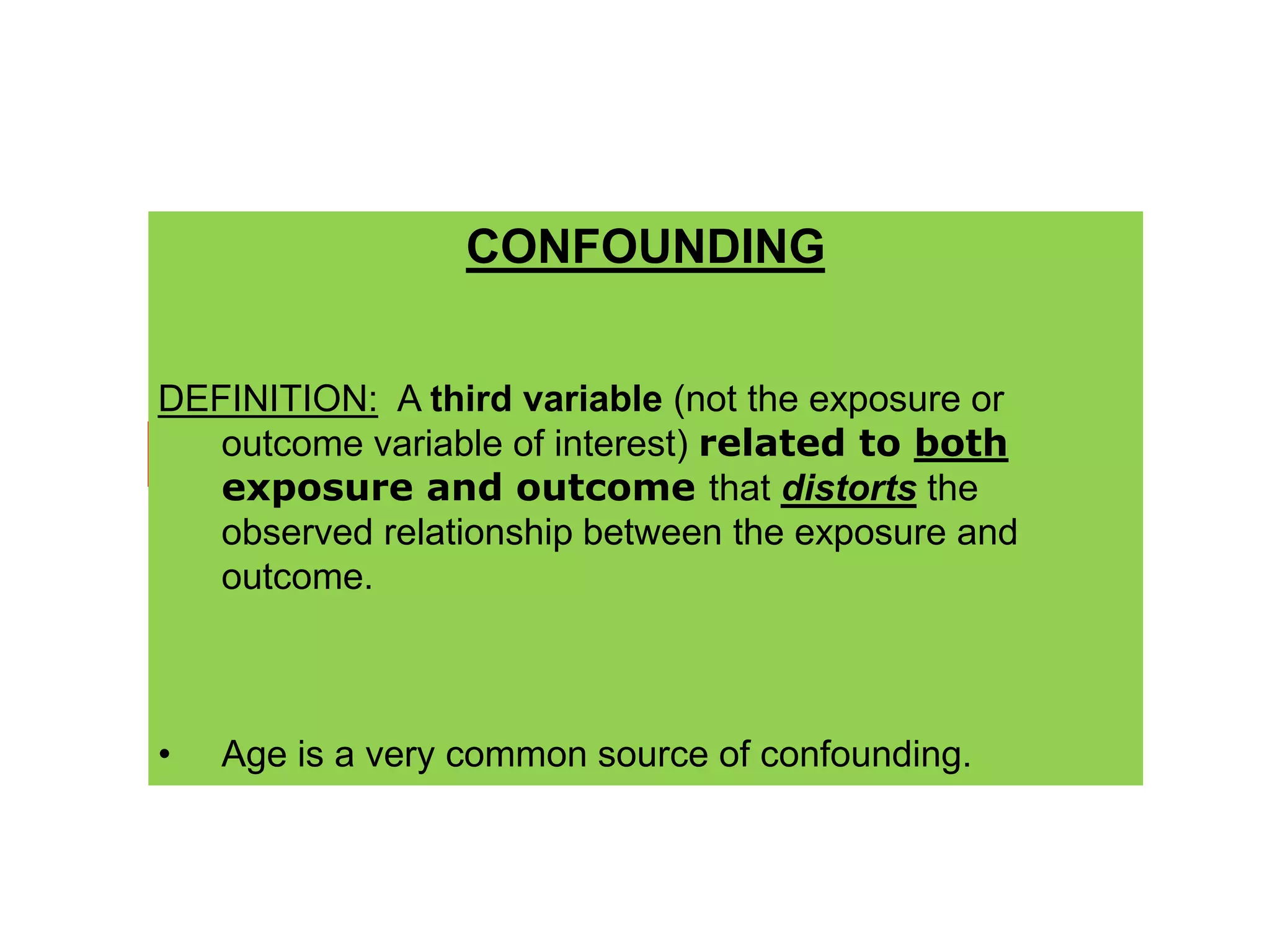
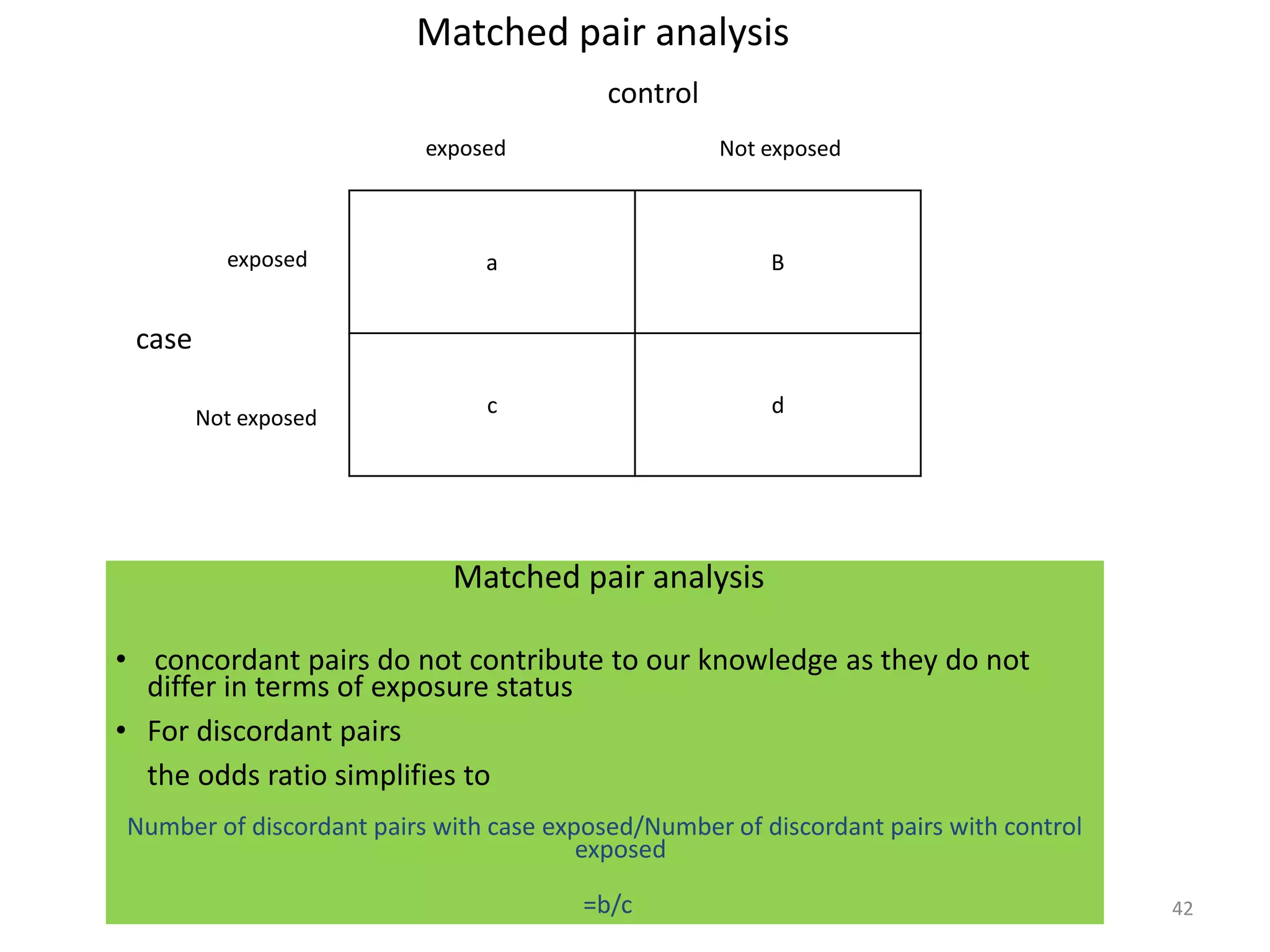
2) Specific types of biases discussed include recall bias, interviewer bias, observer bias, non-response bias, inappropriate control selection, incidence-prevalence bias, loss to follow up, migration bias, Berksonian bias, healthy worker effect, exposure-related bias, non-differential misclassification, and differential misclassification.
3) Bias can