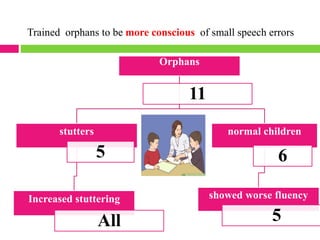
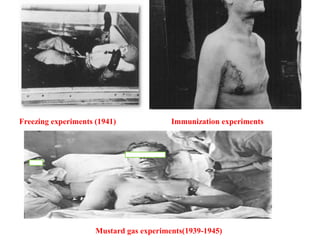
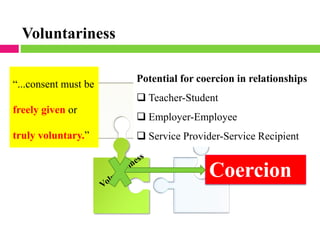
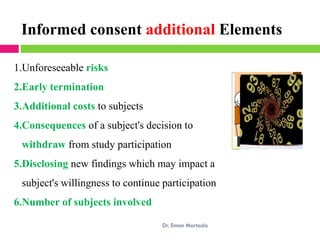
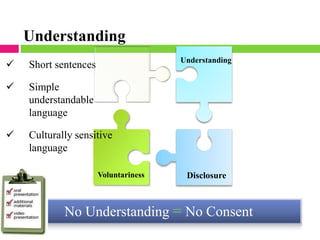
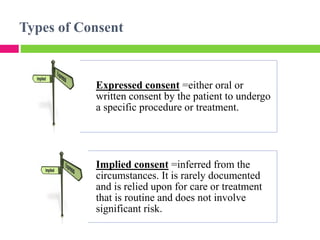
Dr. Eman Mortada discusses research ethics and provides an outline on the topic. The document outlines key concepts such as defining research ethics, the consequences of scientific misconduct, and the need and objectives for research ethics. It also provides a historical perspective on unethical practices through examples like the Tuskegee Syphilis Study and Nazi experiments. The development of ethics codes is reviewed, including the Nuremberg Code, Declaration of Helsinki, and Belmont Report. Ethical principles and dilemmas in research are also discussed.