Embed presentation
Downloaded 26 times









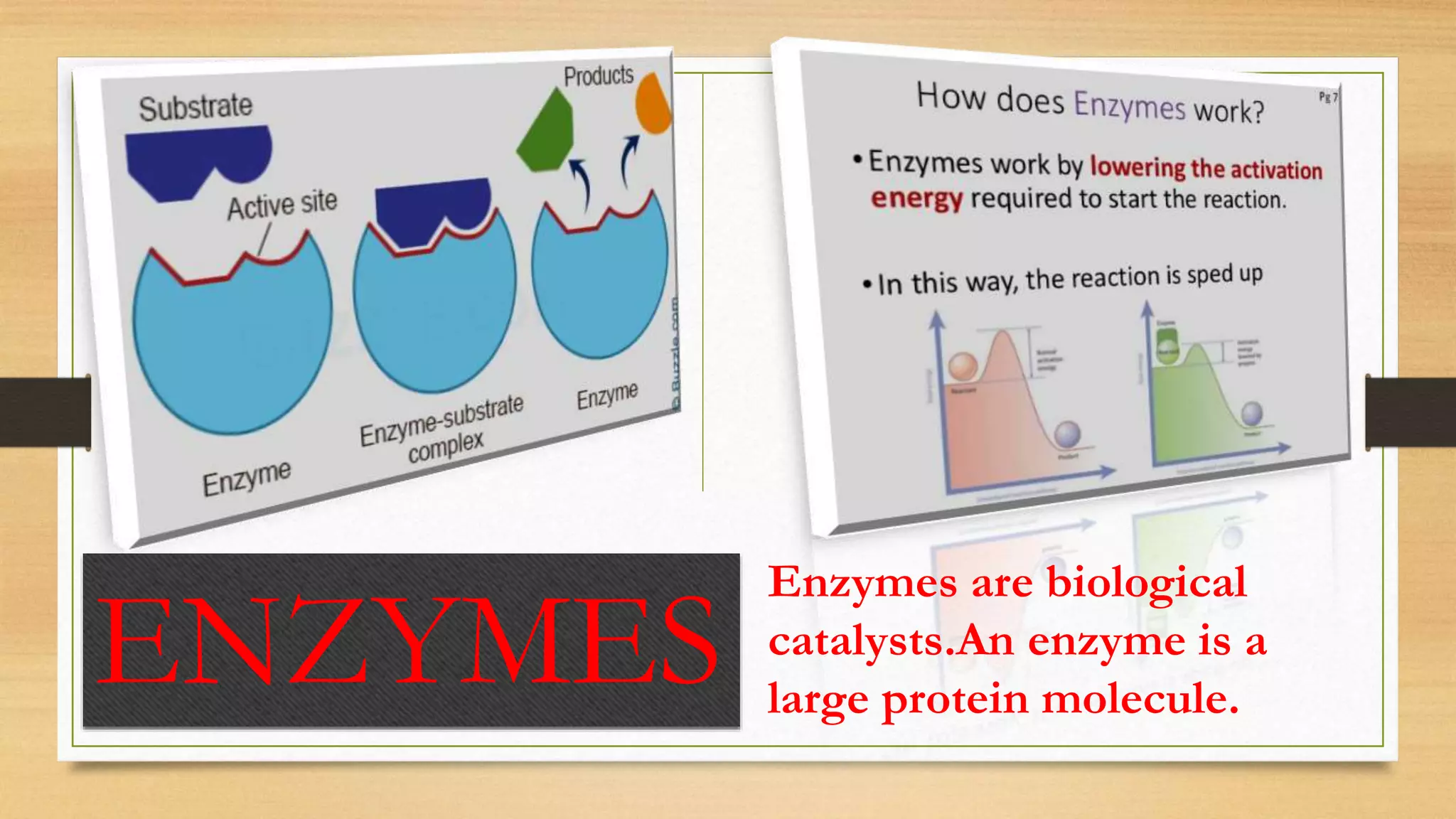


Catalysts speed up chemical reactions by lowering the activation energy required for both the forward and reverse reactions. Catalysts do this by changing the reaction pathway to one that requires less energy. They remain unchanged at the end of the reaction. Catalysts are important in many areas of life, such as in the Haber process for manufacturing ammonia which uses an iron catalyst. Enzymes are biological catalysts that are denatured and lose their catalytic properties at high temperatures due to changes in their protein structure.











