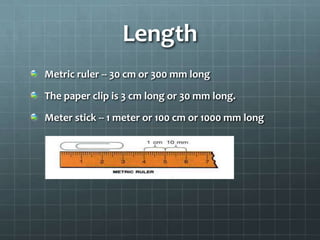
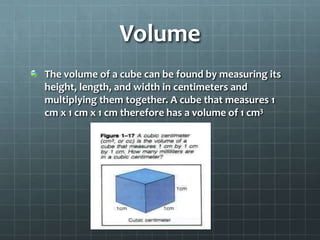
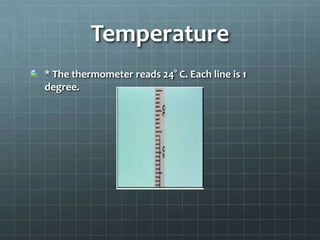
The document discusses various standards of measurement used in science. It describes the metric system including common units of length, volume, mass, density, time, and temperature. For length, the basic metric unit is the meter. For volume, the basic unit is the liter. For mass, the basic unit is the kilogram. Density is a derived unit that represents mass per unit volume. Temperature is measured on the Kelvin scale in the SI system, with Celsius degrees also discussed.