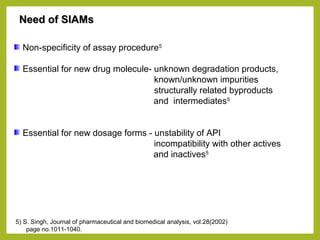
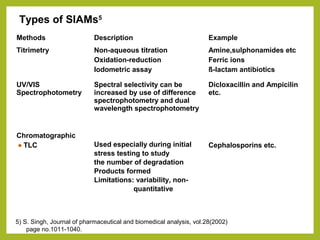
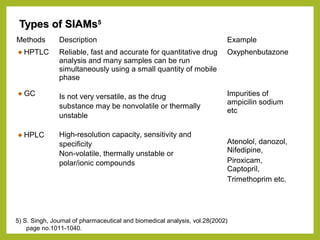
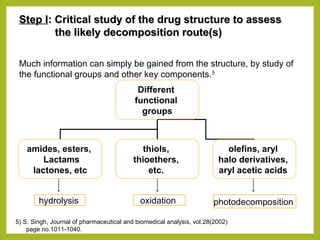
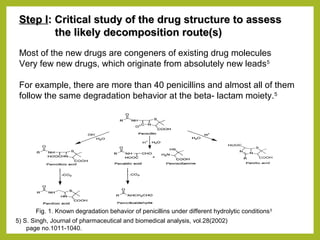
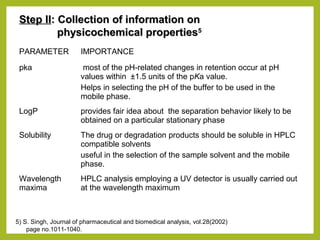
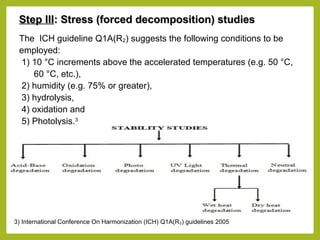
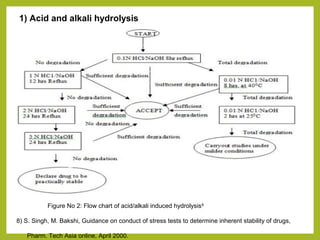
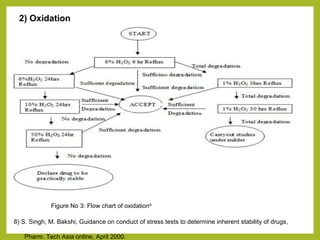
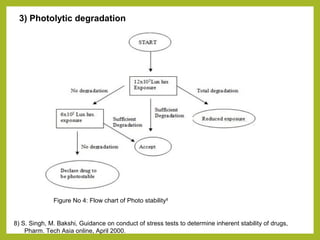
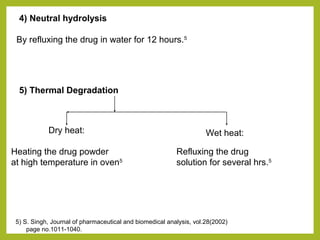
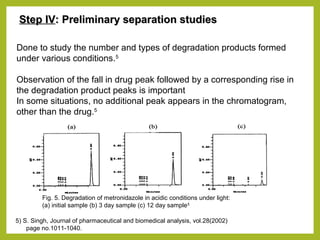
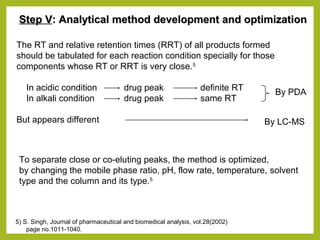
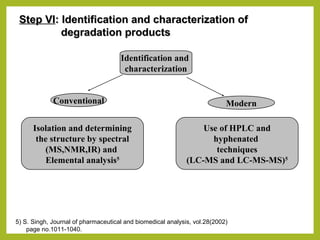
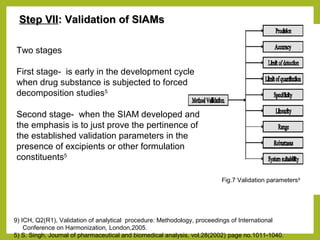
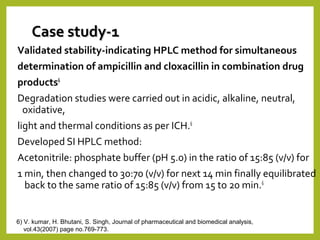
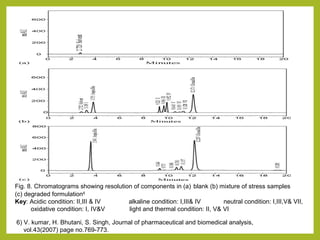
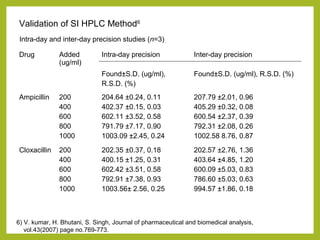
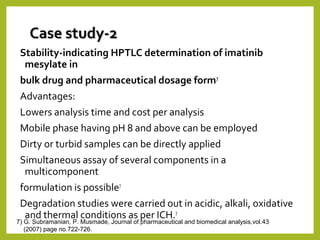
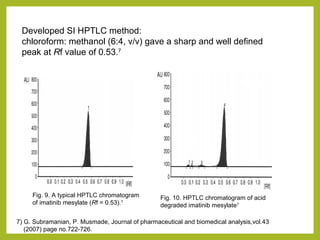
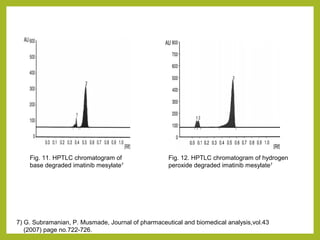
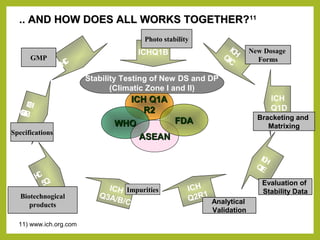
The document discusses stability studies of pharmaceuticals. It begins with an introduction defining stability studies and their purpose to ensure drug efficacy, safety and quality over the shelf life. It then outlines the key stages of stability studies including stress testing, international stability guidelines, and development of stability indicating assay methods (SIAMs). SIAMs are validated quantitative methods that can detect changes in drugs and products over time. The document concludes with two case studies demonstrating the development and validation of SIAMs for pharmaceutical combinations using HPLC and HPTLC methods.






![STABILITY INDICATINGSTABILITY INDICATING
ASSAY METHODSASSAY METHODS
[SIAMs][SIAMs]](https://image.slidesharecdn.com/stabilityindicatingassay-190509150924/85/Stability-indicating-assay-8-320.jpg)