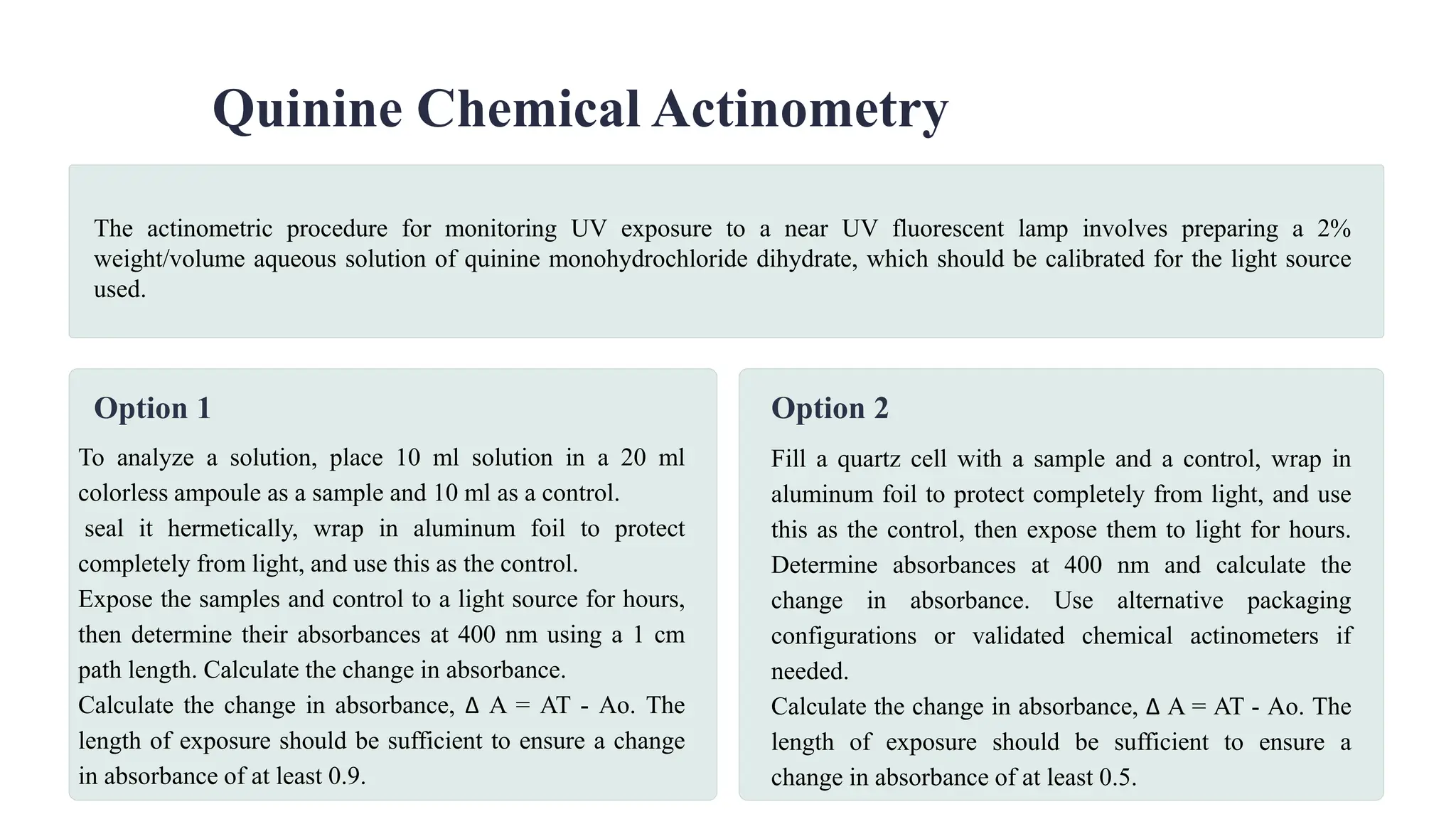
The document outlines the ICH guideline Q1B for photostability testing of new drug substances and products, highlighting the importance of evaluating their intrinsic photostability characteristics. It specifies the testing procedures, conditions, and requirements, such as the use of specific light sources and the need for confirmatory studies. Additionally, it discusses sample presentation, analysis, and the need for special precautions for light-sensitive products.