Embed presentation
Downloaded 15 times


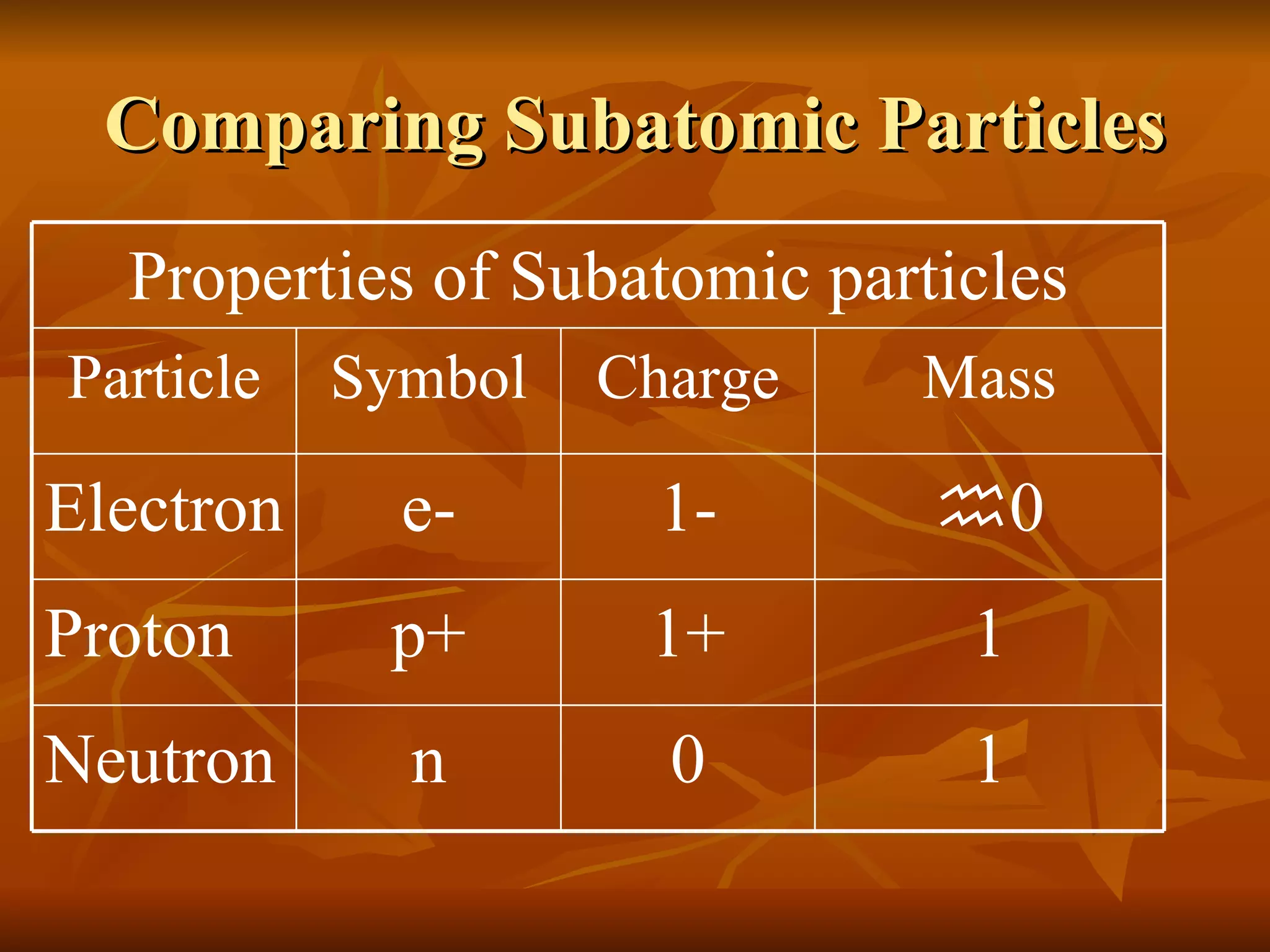
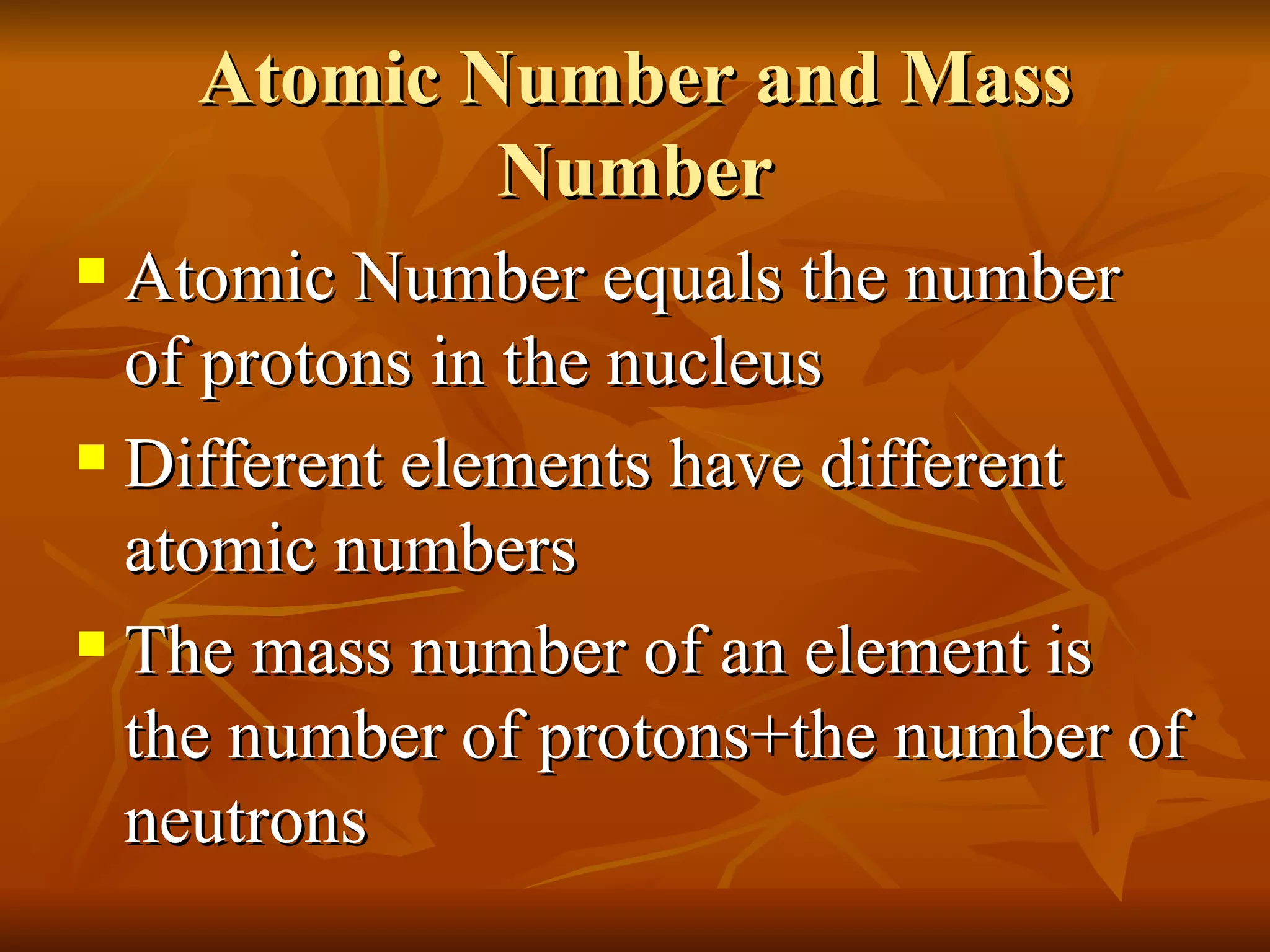


The document discusses the structure of atoms and properties of subatomic particles. It defines protons, neutrons, and electrons, noting their charges and locations. It explains that the atomic number is the number of protons and determines the element, while the mass number is the total of protons and neutrons. Isotopes are defined as atoms of the same element that have different numbers of neutrons and mass numbers.





