Embed presentation
Download to read offline




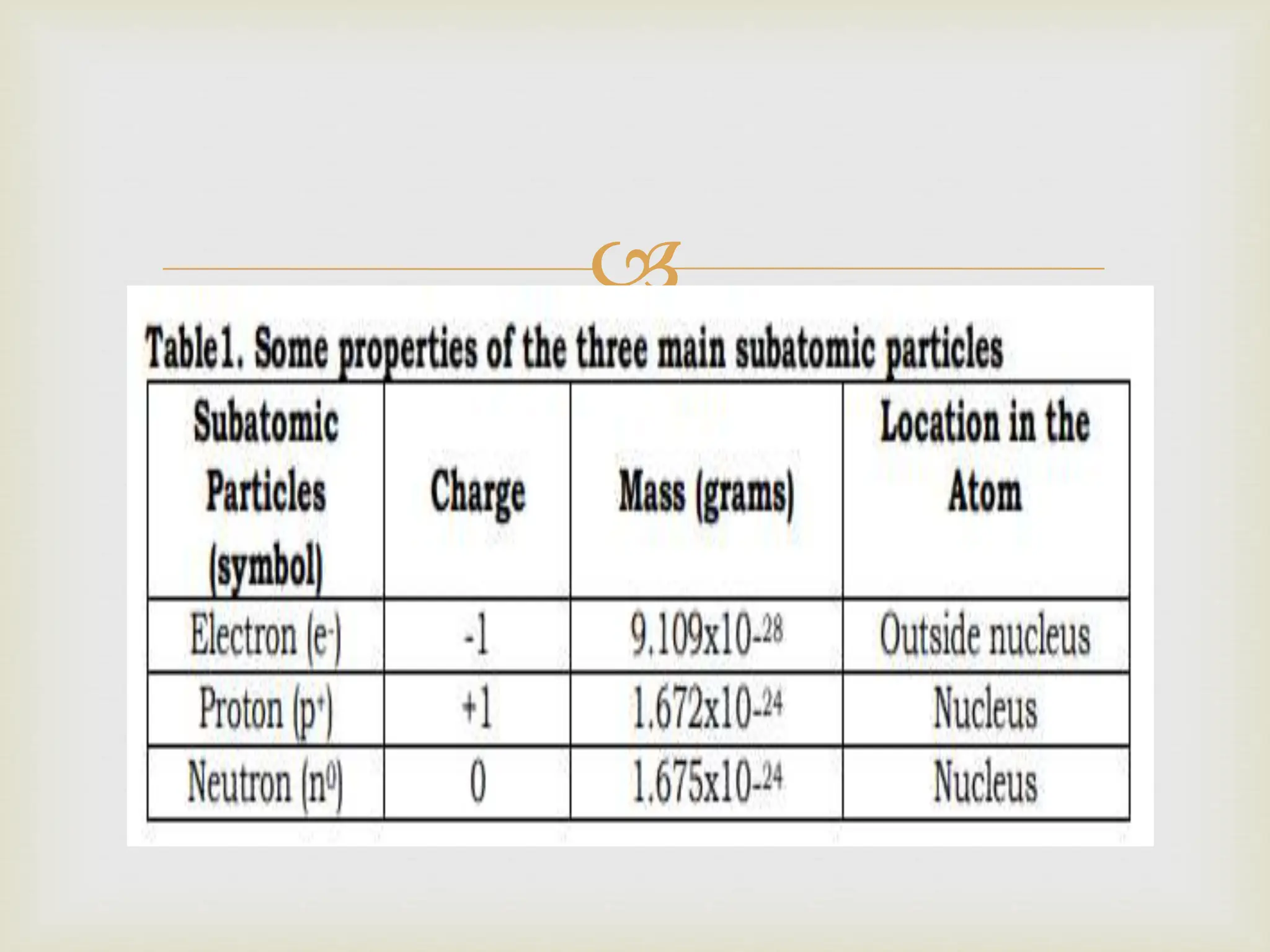




Atoms contain positively charged protons and neutrons located in the nucleus at the center. Negatively charged electrons orbit the nucleus. Atoms are electrically neutral when they contain an equal number of protons and electrons. Protons and neutrons have similar masses and are found in the nucleus, while electrons are much lighter and located outside the nucleus. The atomic number identifies the number of protons and electrons in a neutral atom, while the mass number is the total of protons and neutrons in the nucleus.








