Embed presentation
Downloaded 10 times
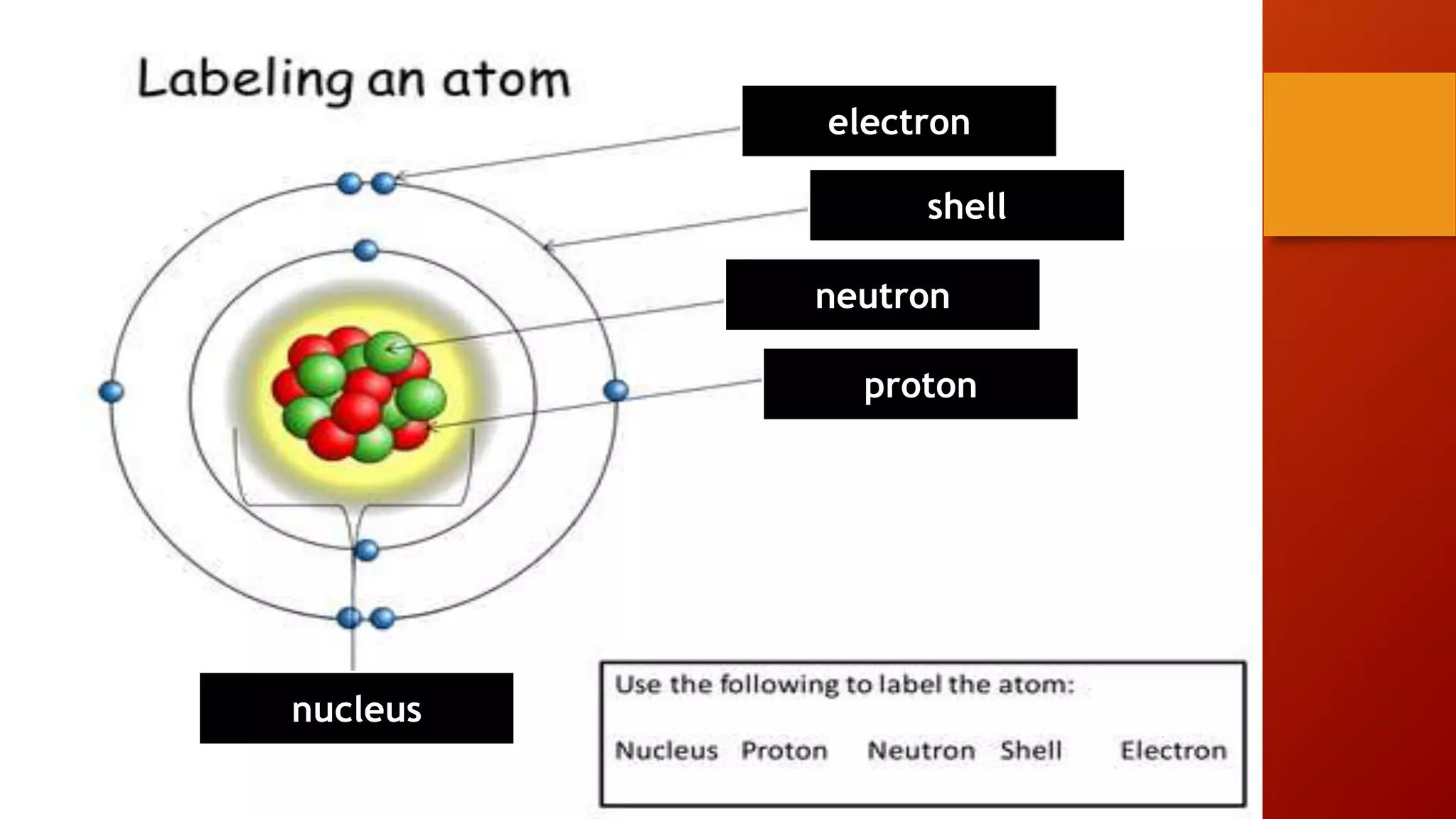
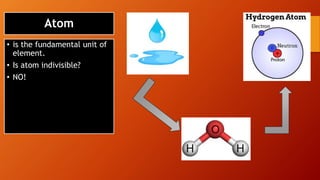
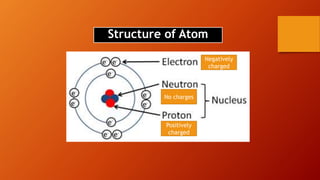
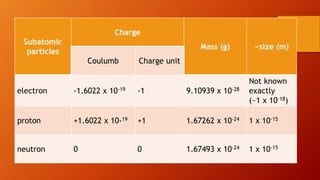
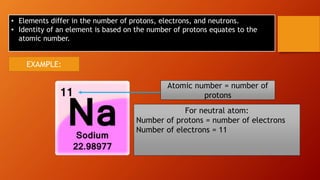
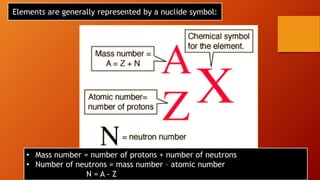
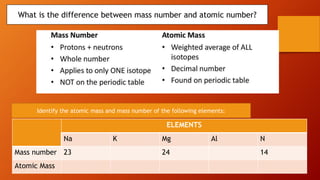
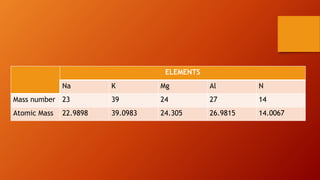
This document discusses the structure of atoms and subatomic particles. It explains that atoms were once thought to be indivisible but are actually made up of subatomic particles, including electrons, protons, and neutrons. Each element is defined by its atomic number, or number of protons, while the mass number refers to the total number of protons and neutrons. Key subatomic particles are electrons, which have a negative charge; protons, which have a positive charge; and neutrons, which have no charge.








