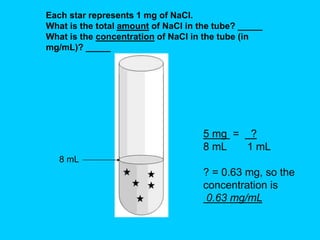
This document provides instructions and definitions for preparing chemical solutions and reagents. It discusses the types of vessels used to hold solutions, defines key terms like solutes, solvents, concentration, and amount. It also covers how to make solutions of different concentrations by mass, volume, percentage, molarity and describes how to dilute stock solutions to achieve desired concentrations. The goals are to teach how to properly make and measure solutions for experiments.