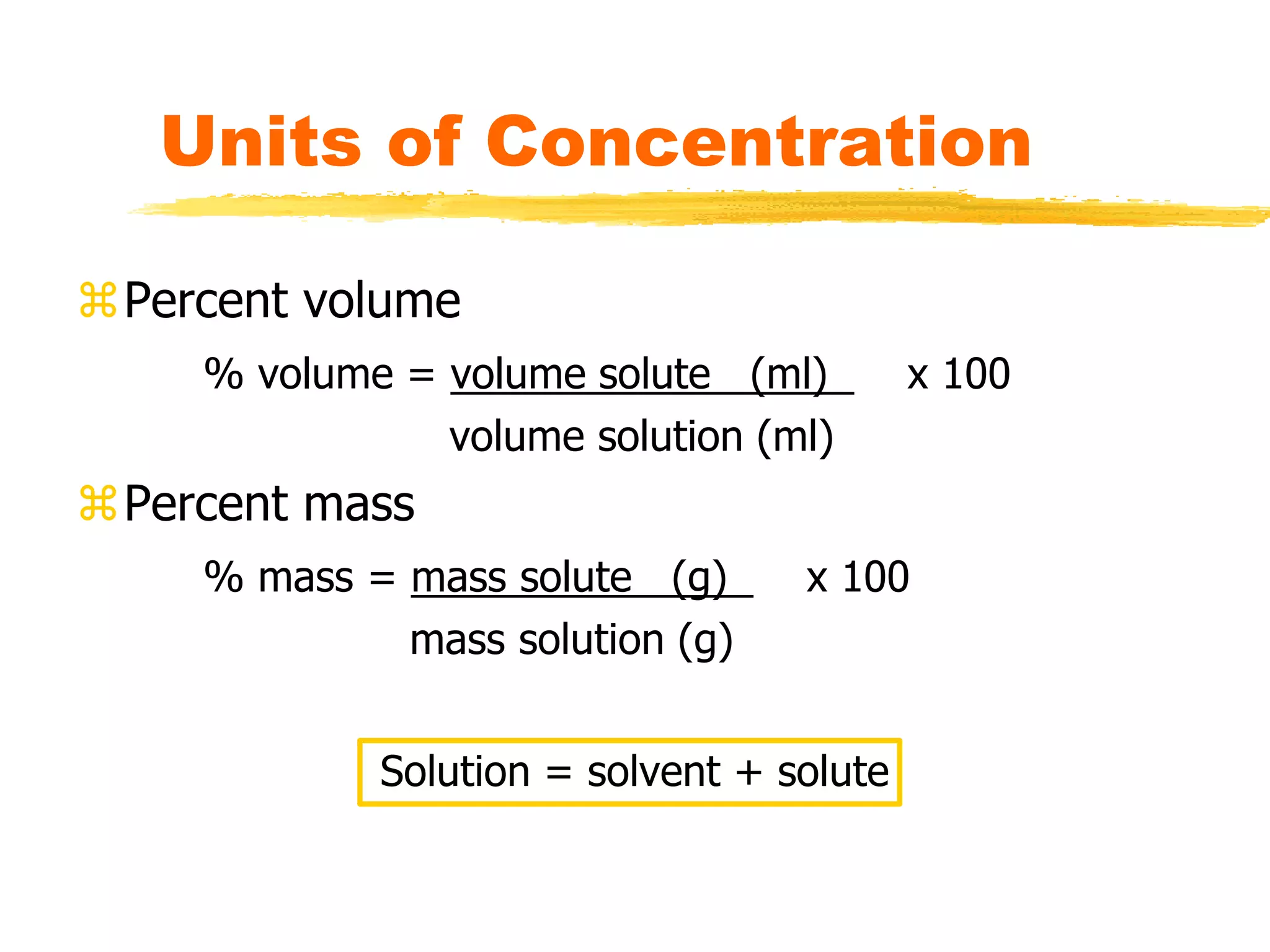
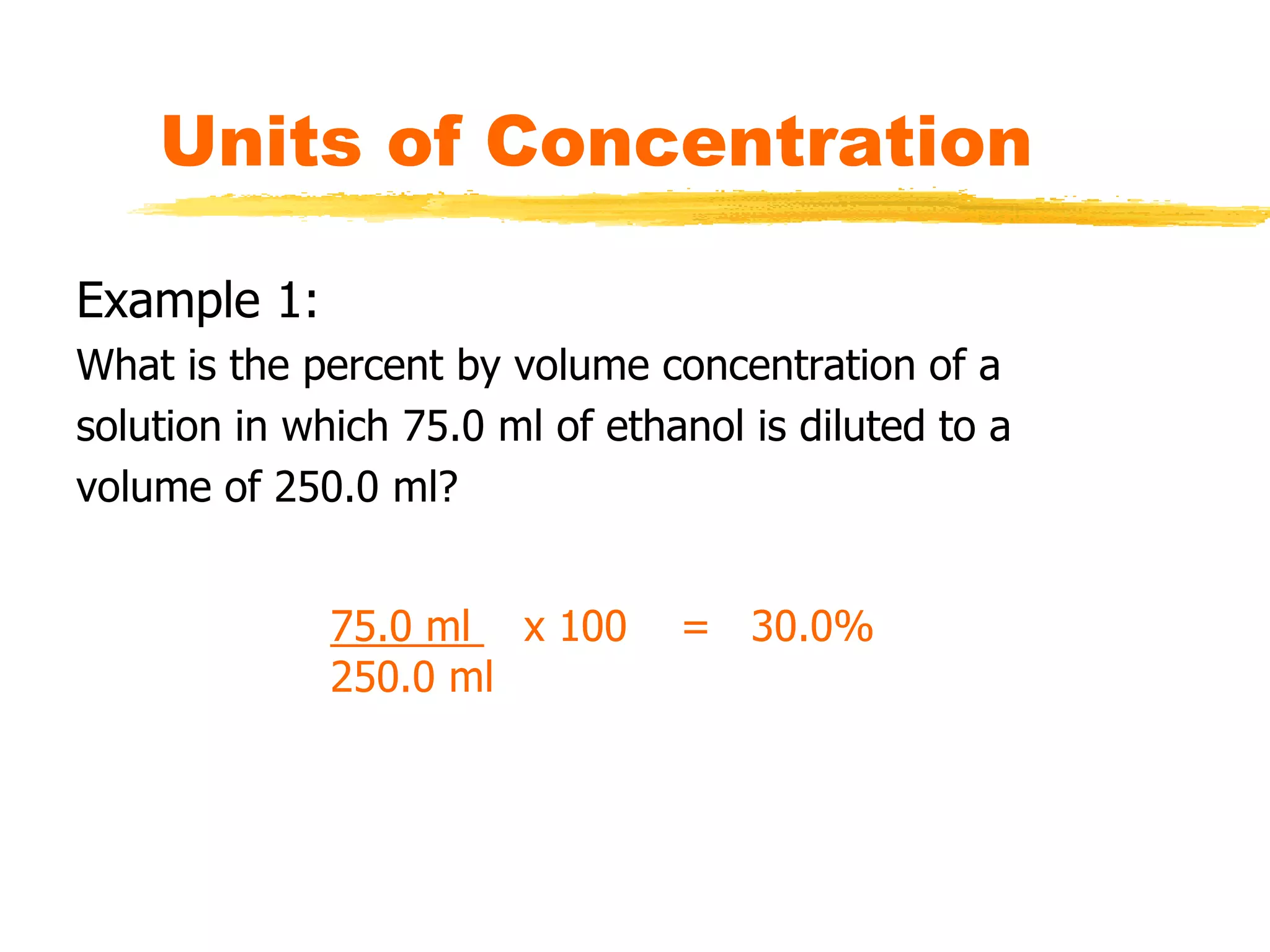
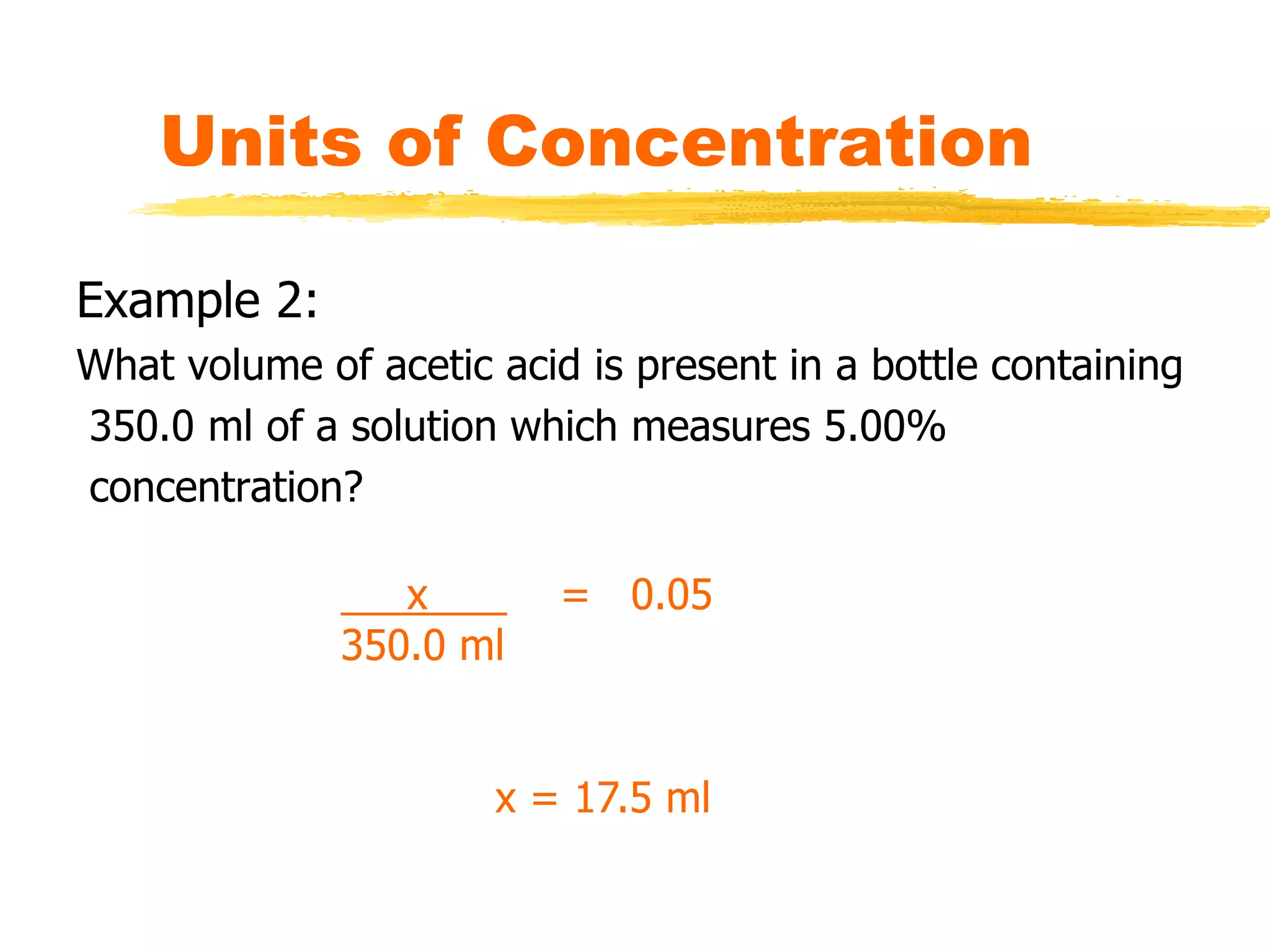
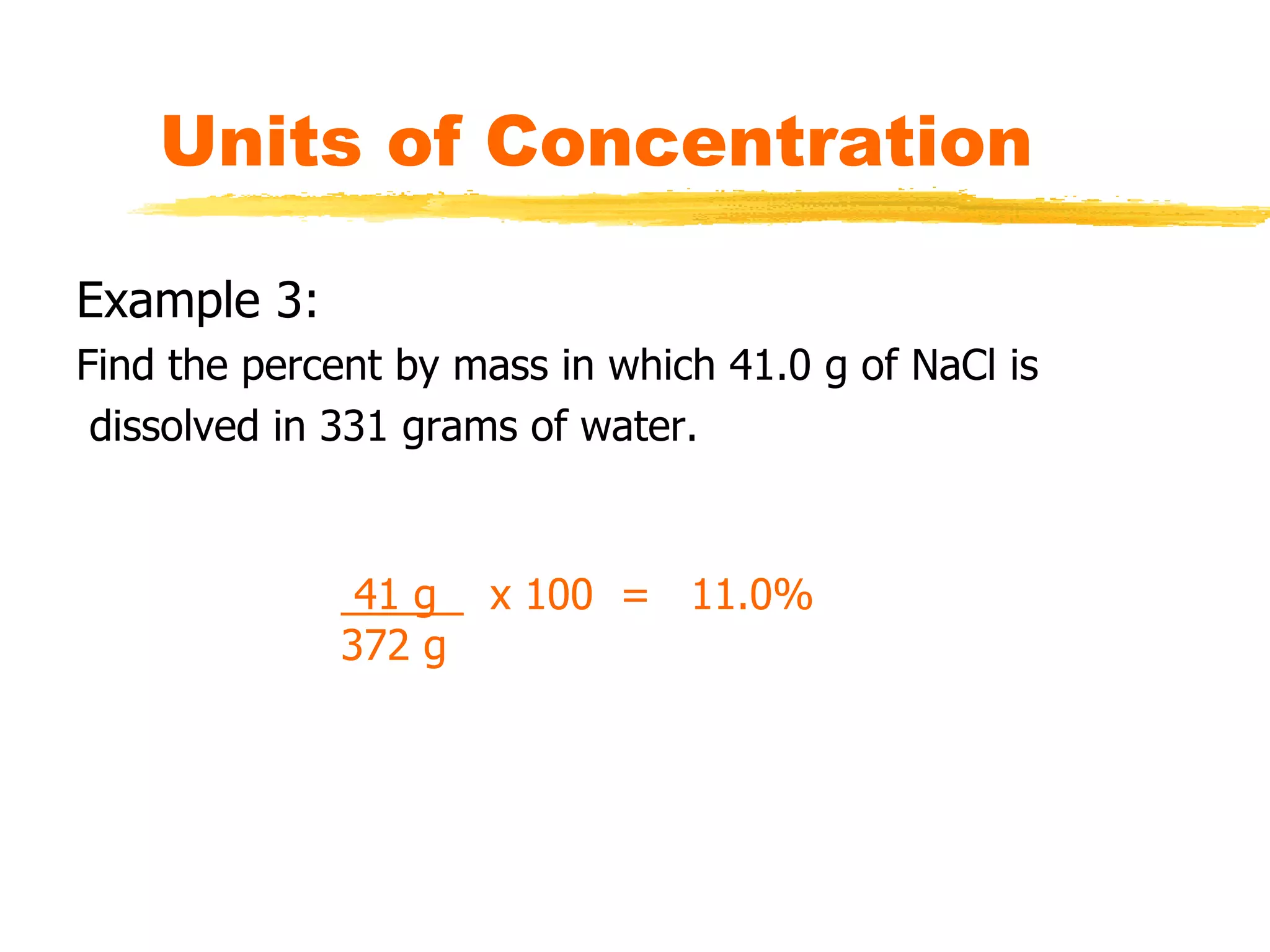
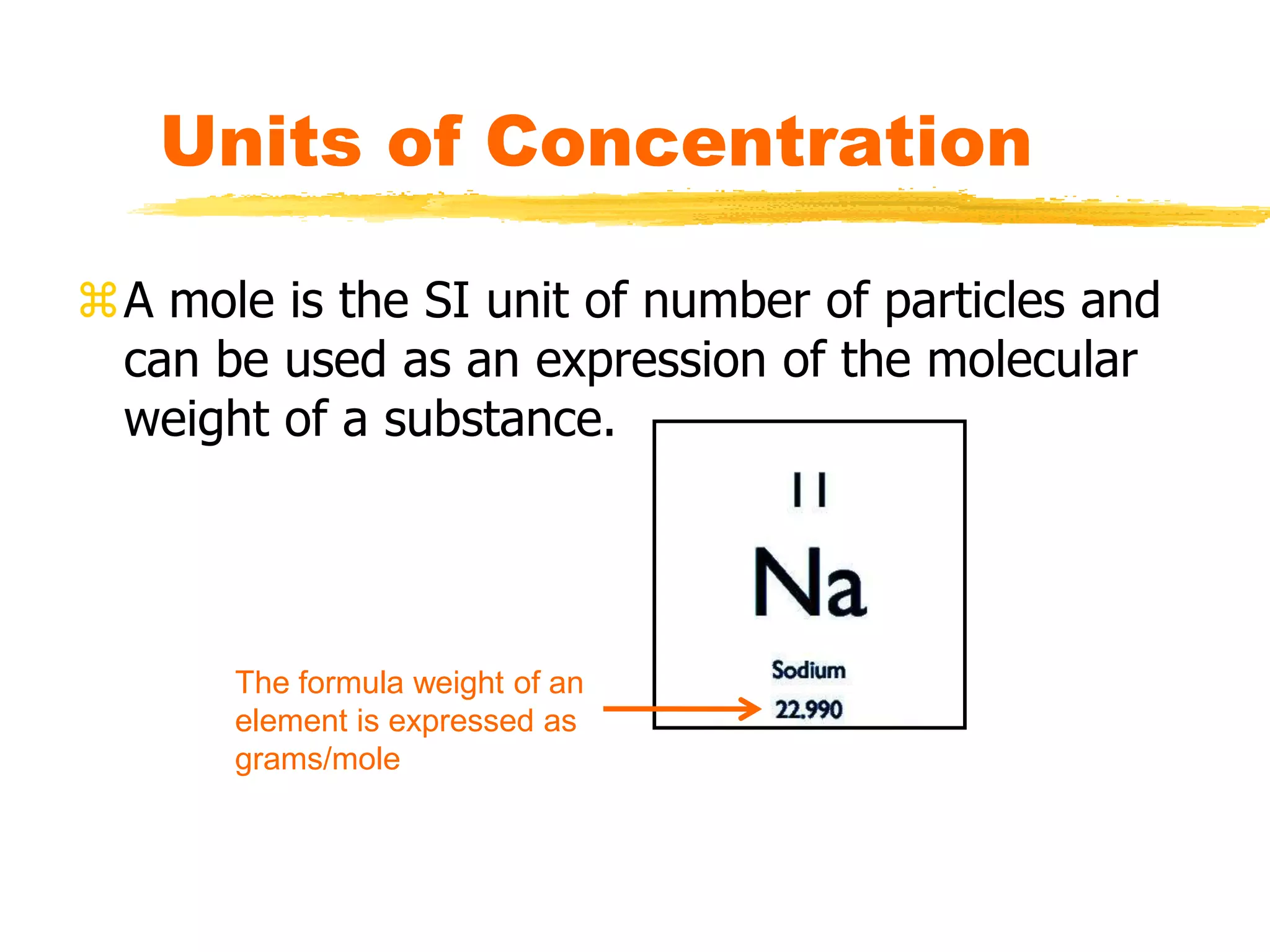
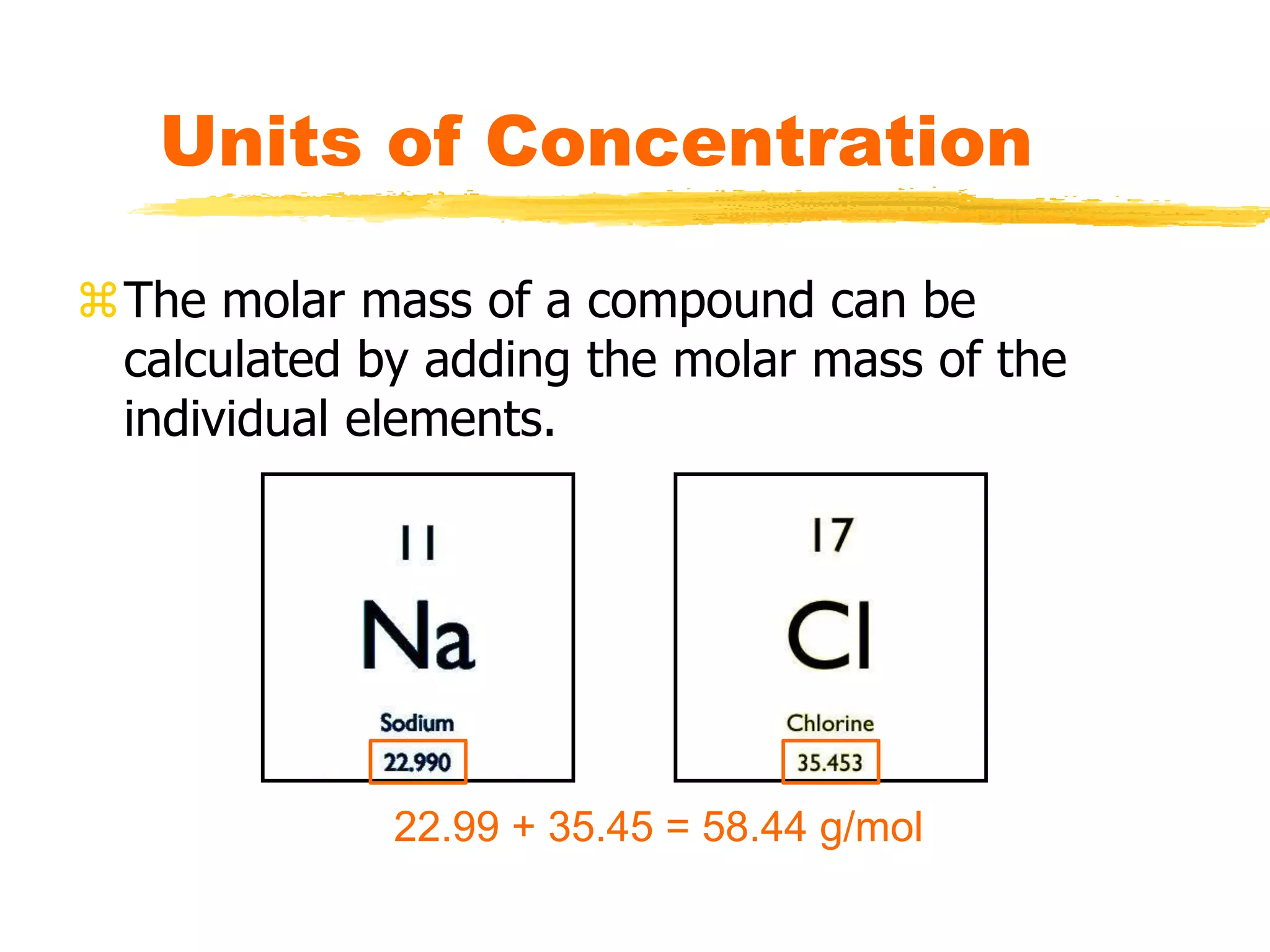
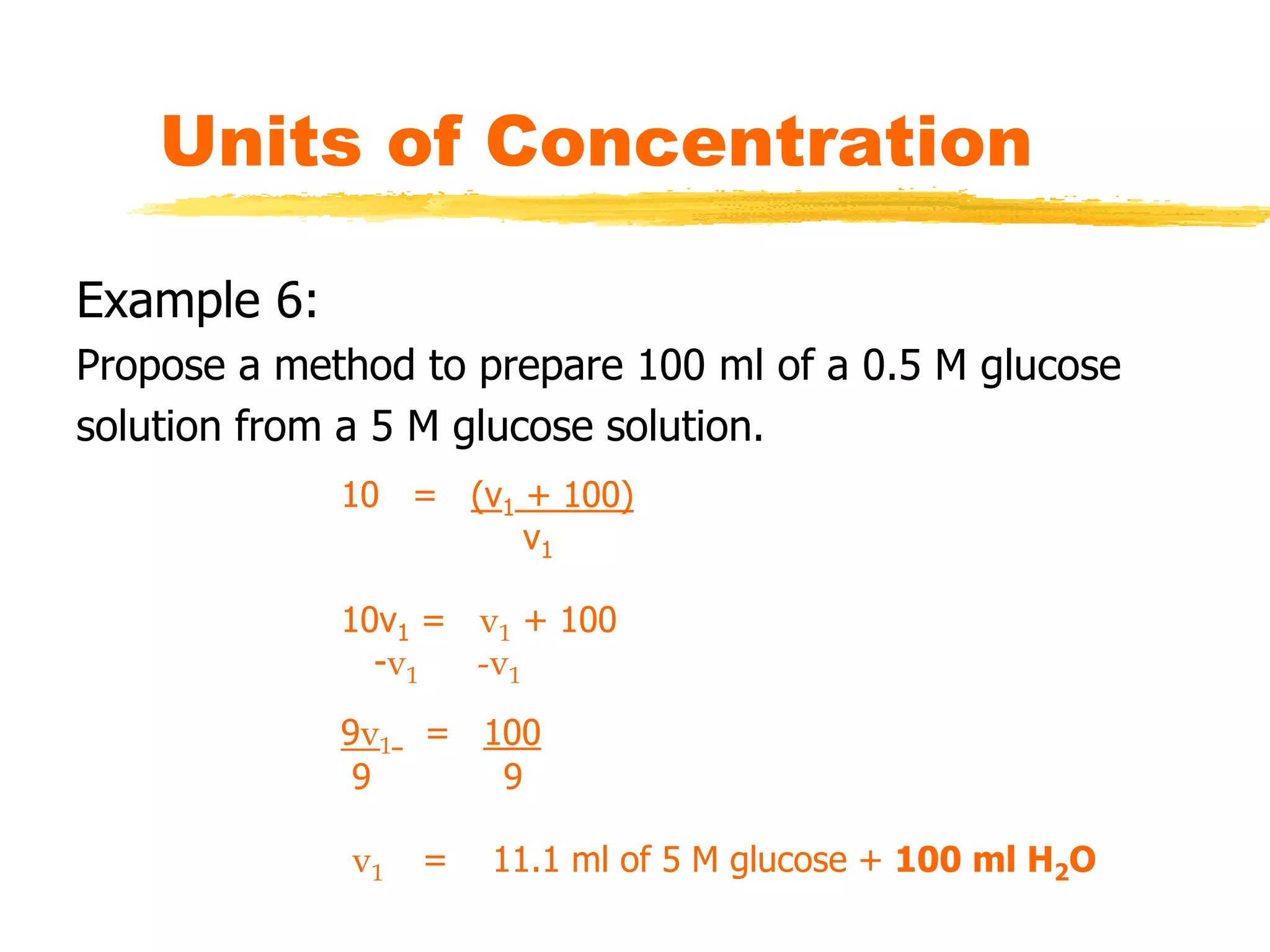
This document discusses units of concentration used to quantify the amount of solute dissolved in a solution. It defines percent by volume and percent by mass as ratios comparing the amount of solute to the total solution. Molarity is introduced as moles of solute per liter of solution, which is a common unit of concentration. Examples are provided for calculating concentration using various units. The document also explains how to calculate the amount of solute needed to make a stock solution of a specific molarity and how to dilute a stock solution to obtain lower concentrations using dilution equations.