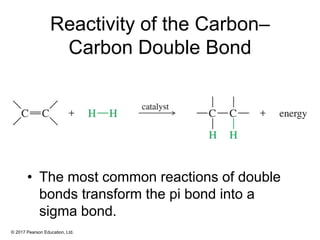
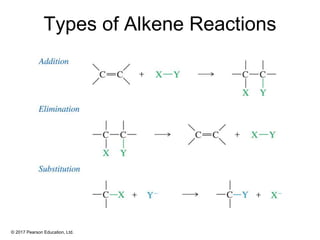
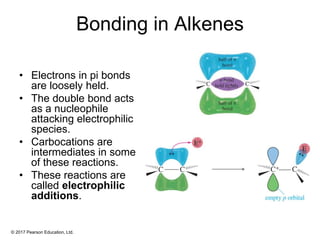
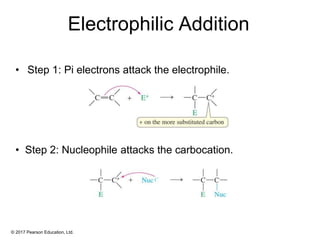
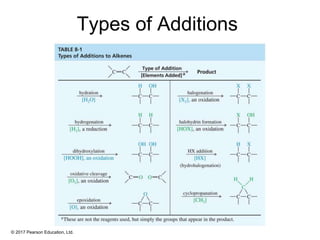
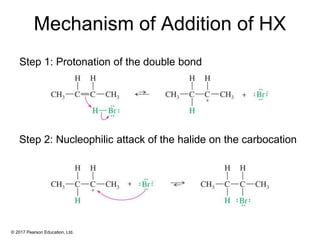
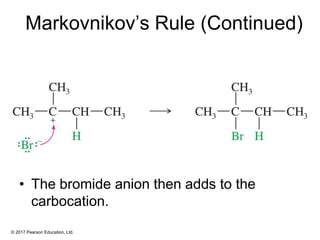
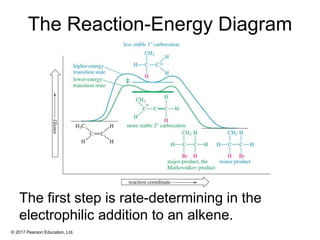
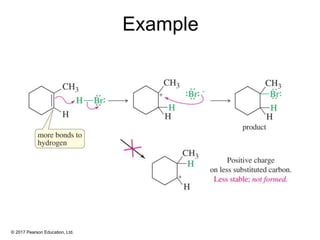
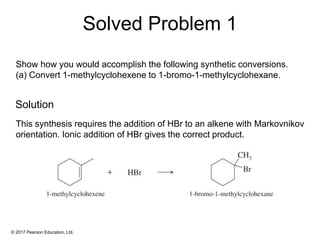
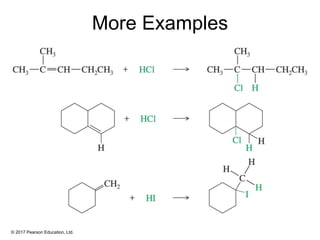
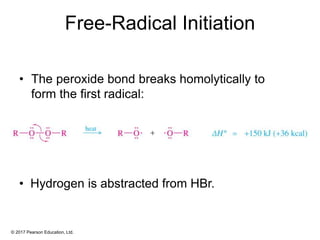
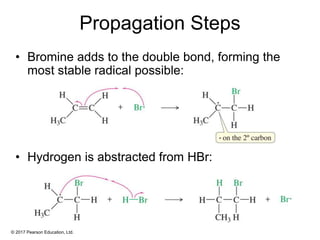
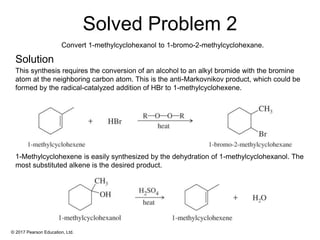
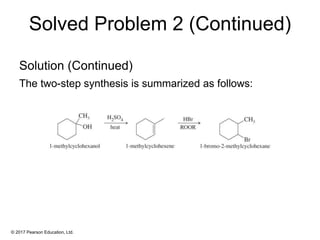
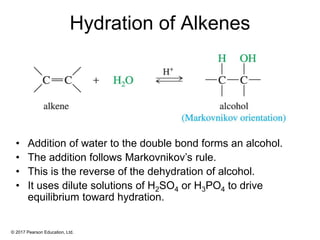
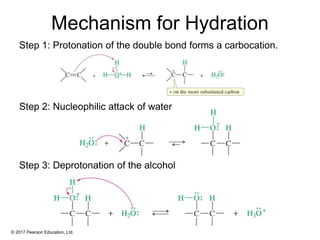
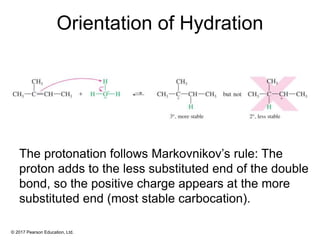
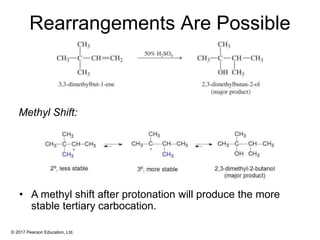
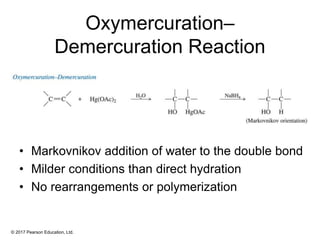
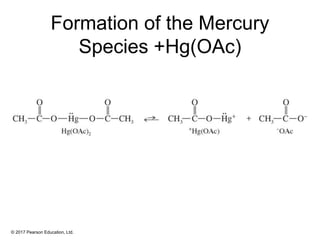
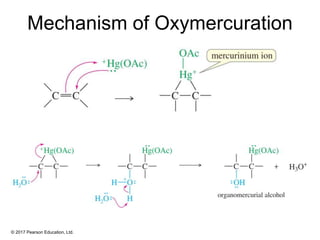
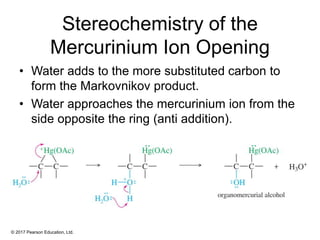
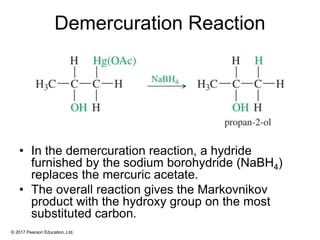
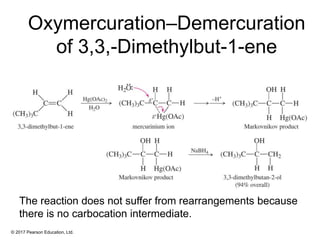
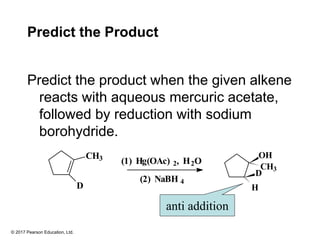
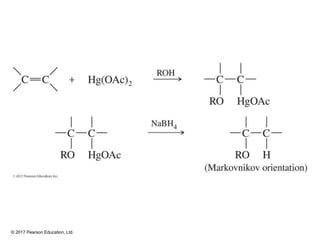
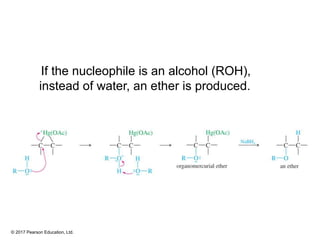
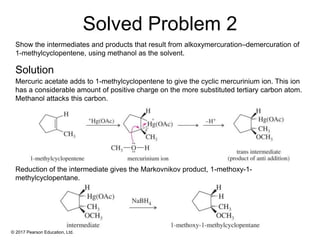
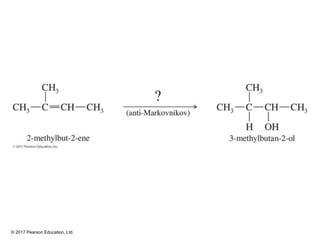
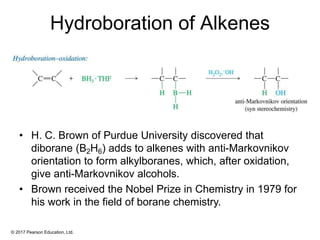
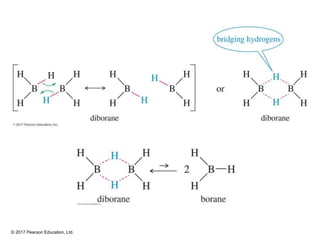
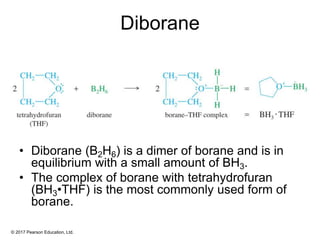
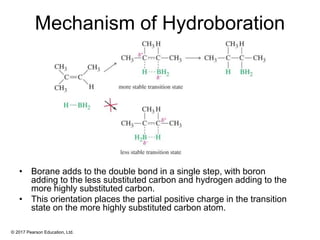
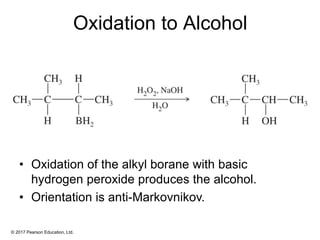
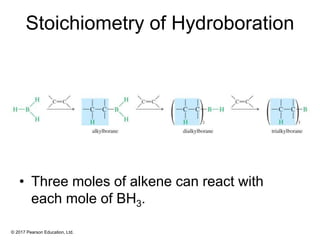
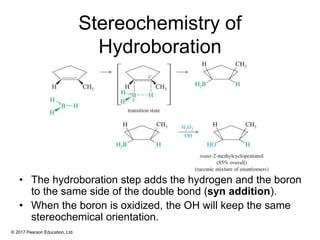
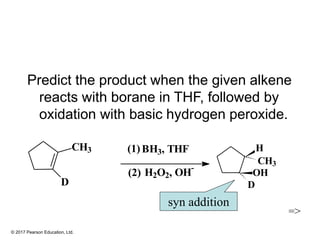
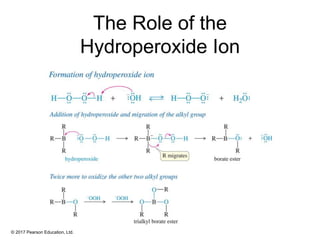
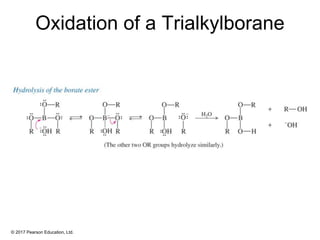
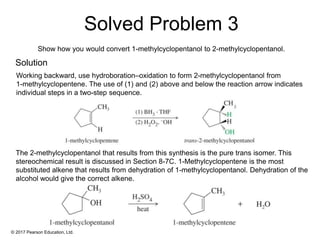
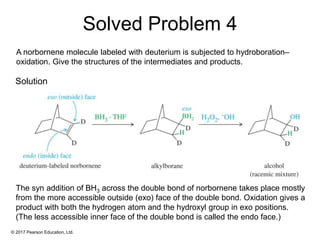
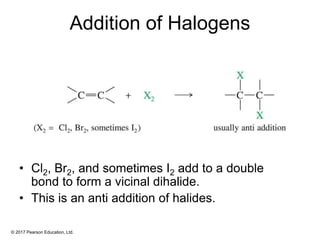
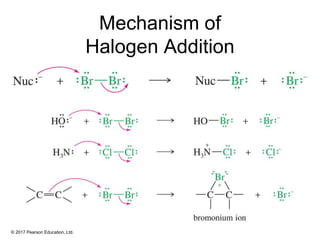
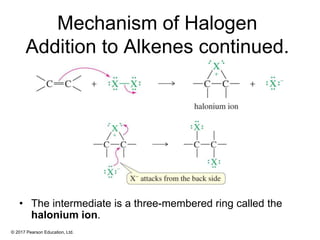
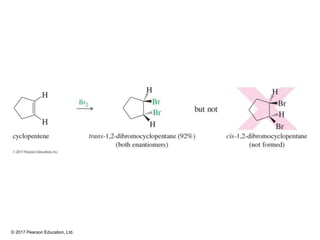
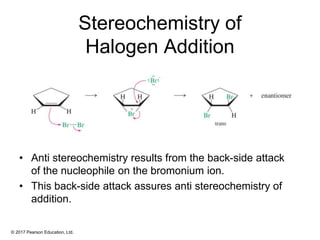
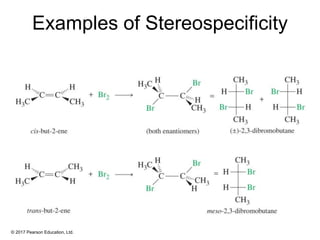
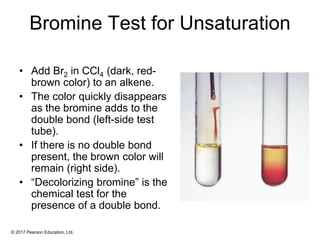
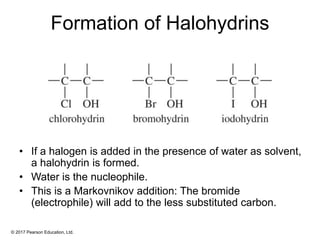
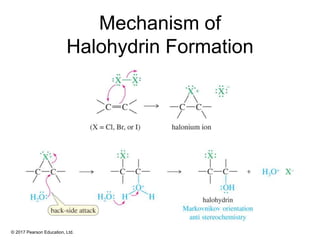
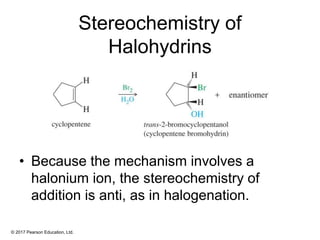
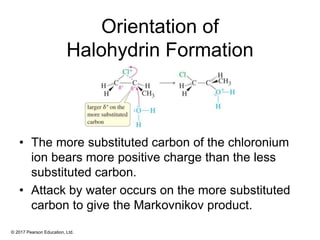
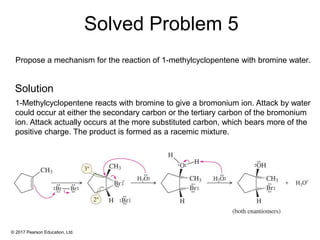
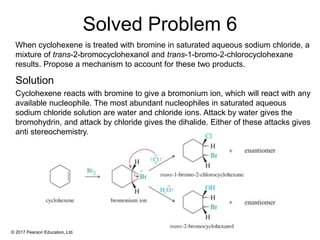
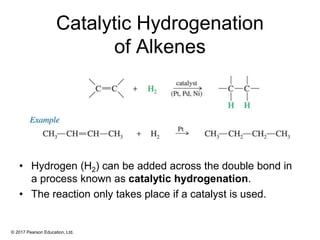
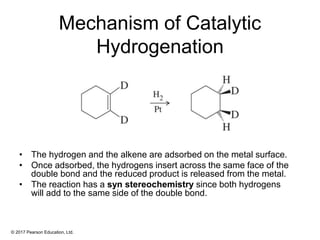
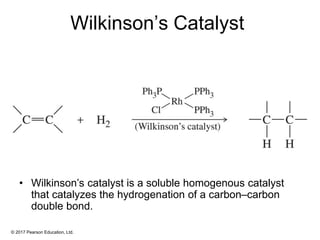
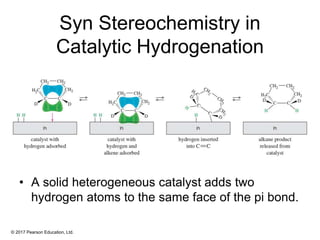
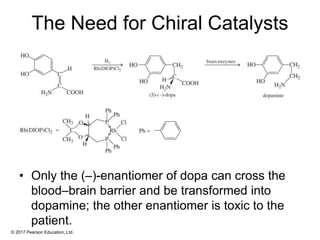
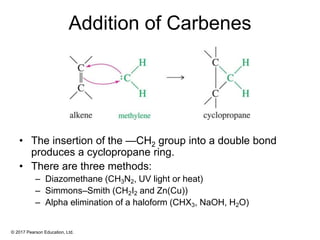
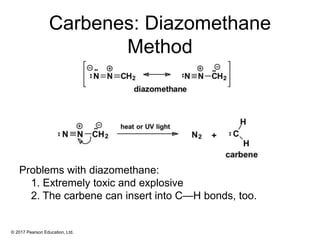
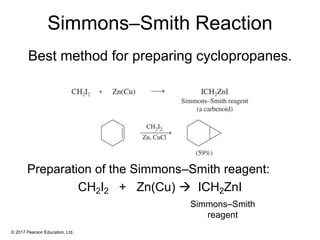
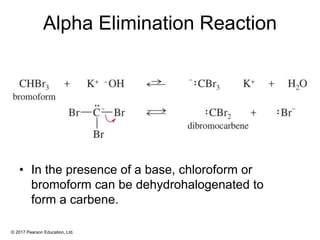
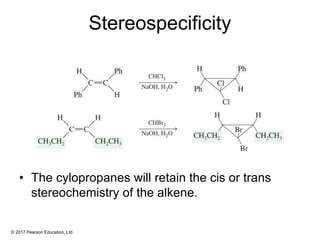
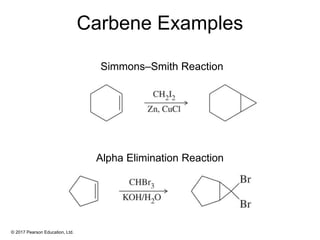
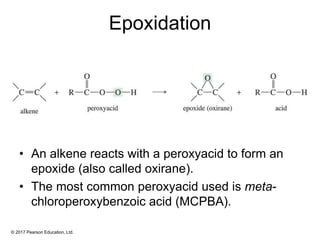
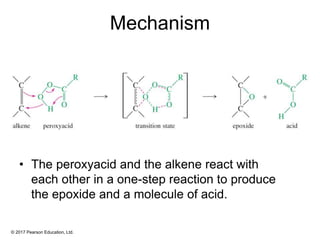
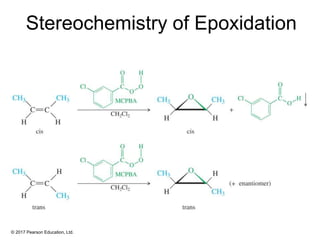
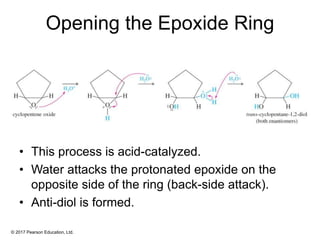
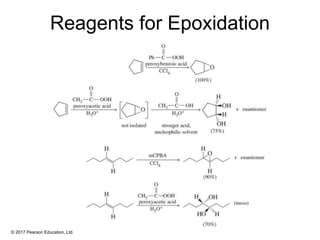
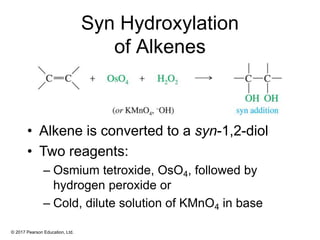
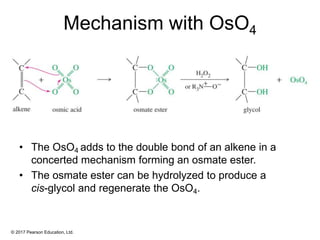
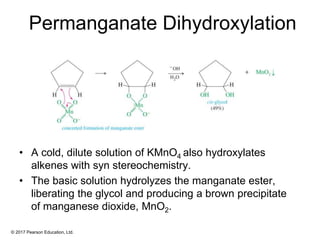
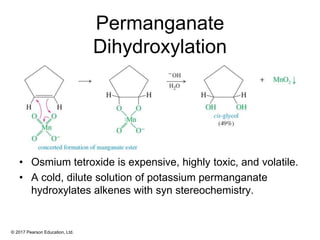
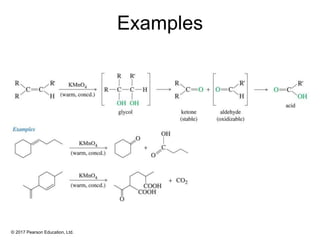
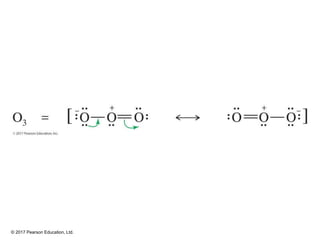
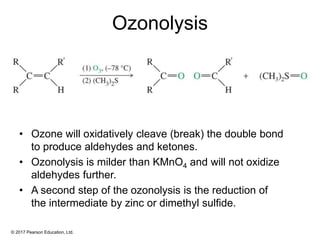
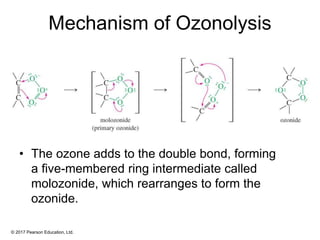
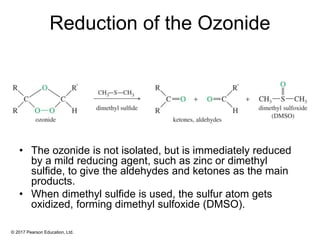
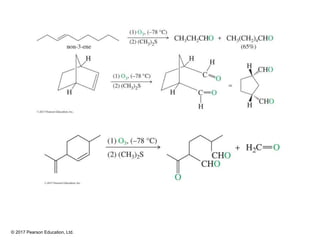
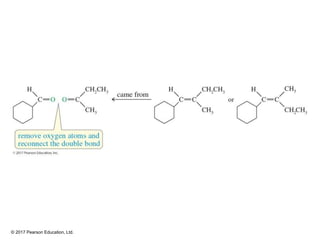
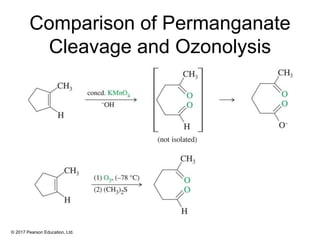
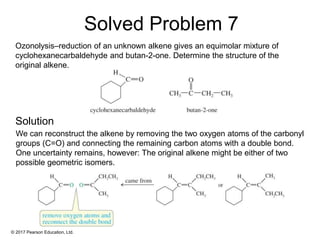
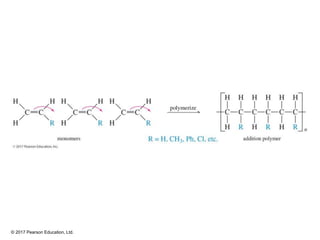
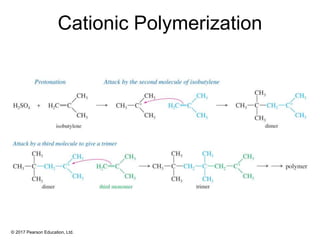
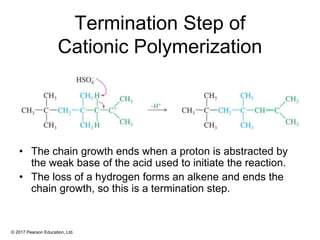
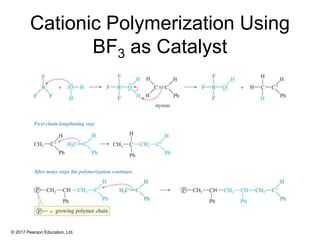
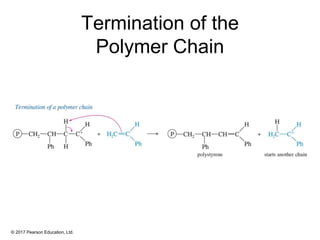
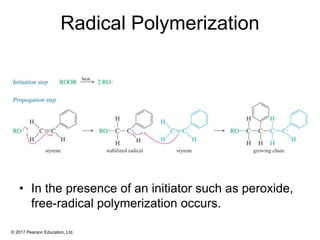
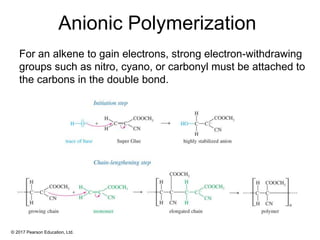
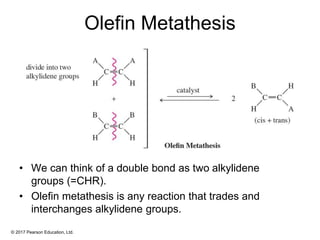
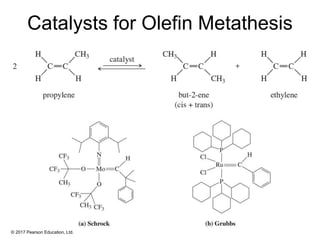
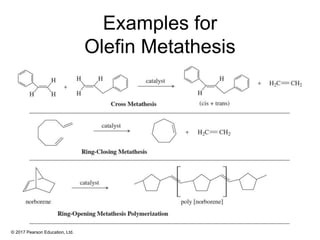
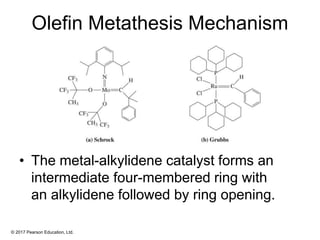
This document provides an overview of reactions of alkenes, including electrophilic addition, hydration, oxymercuration-demercuration, hydroboration-oxidation, and other reactions. It discusses the mechanisms and stereochemistry of these reactions. Key points covered include Markovnikov's rule for electrophilic addition and hydration reactions, the use of mercury compounds and borane to achieve anti-Markovnikov addition, and examples of applying these reactions in synthesis problems.