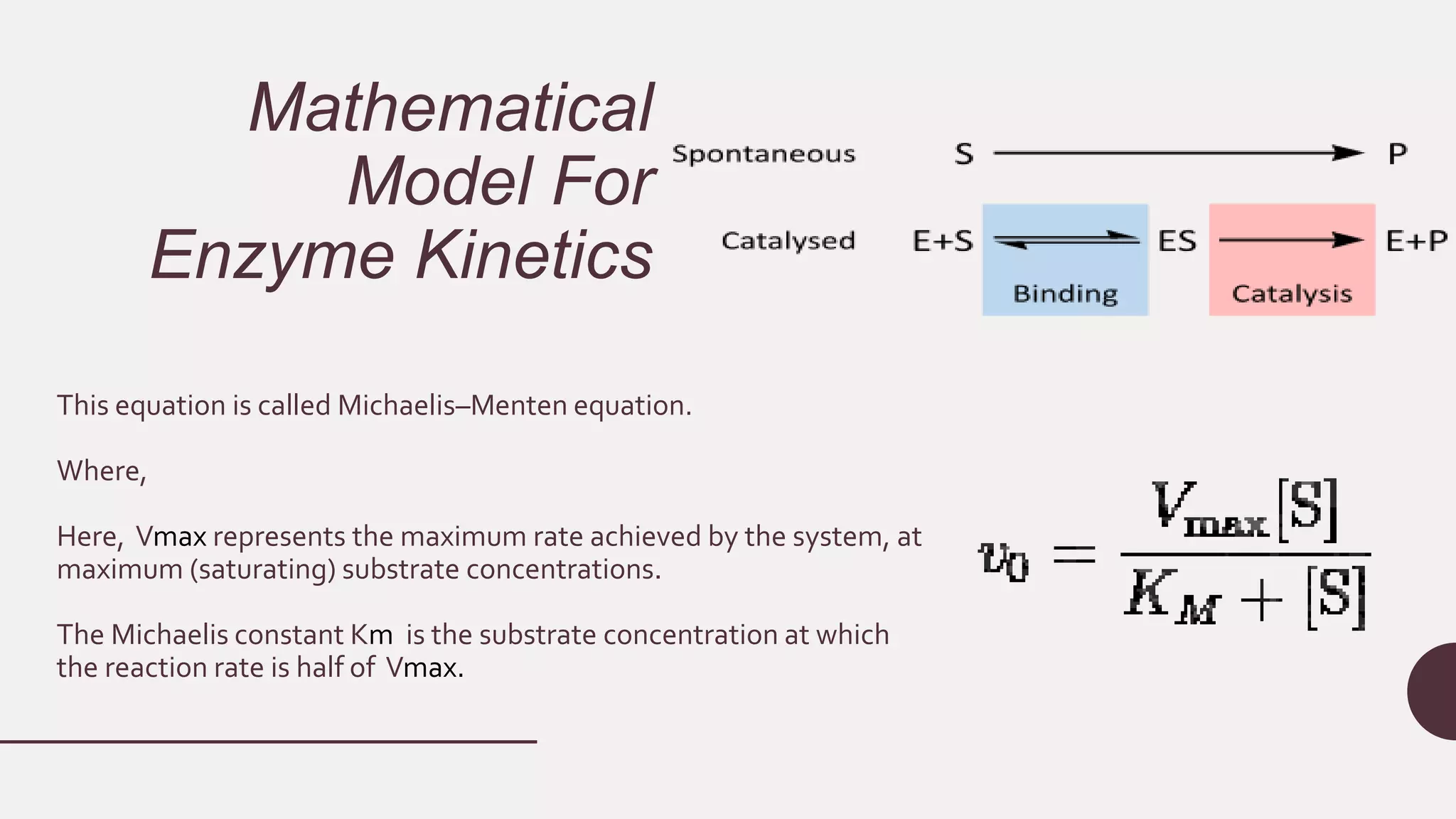
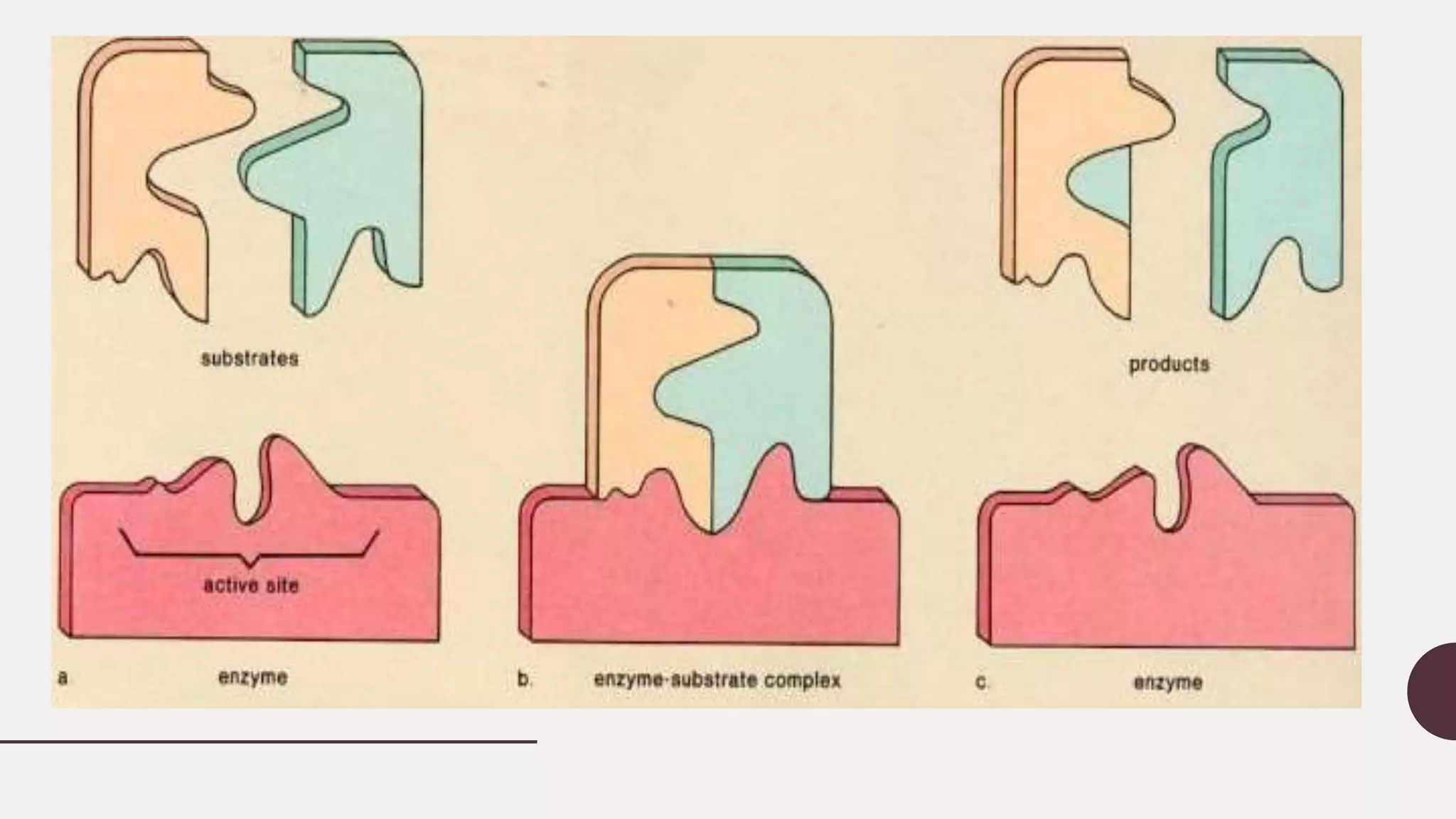
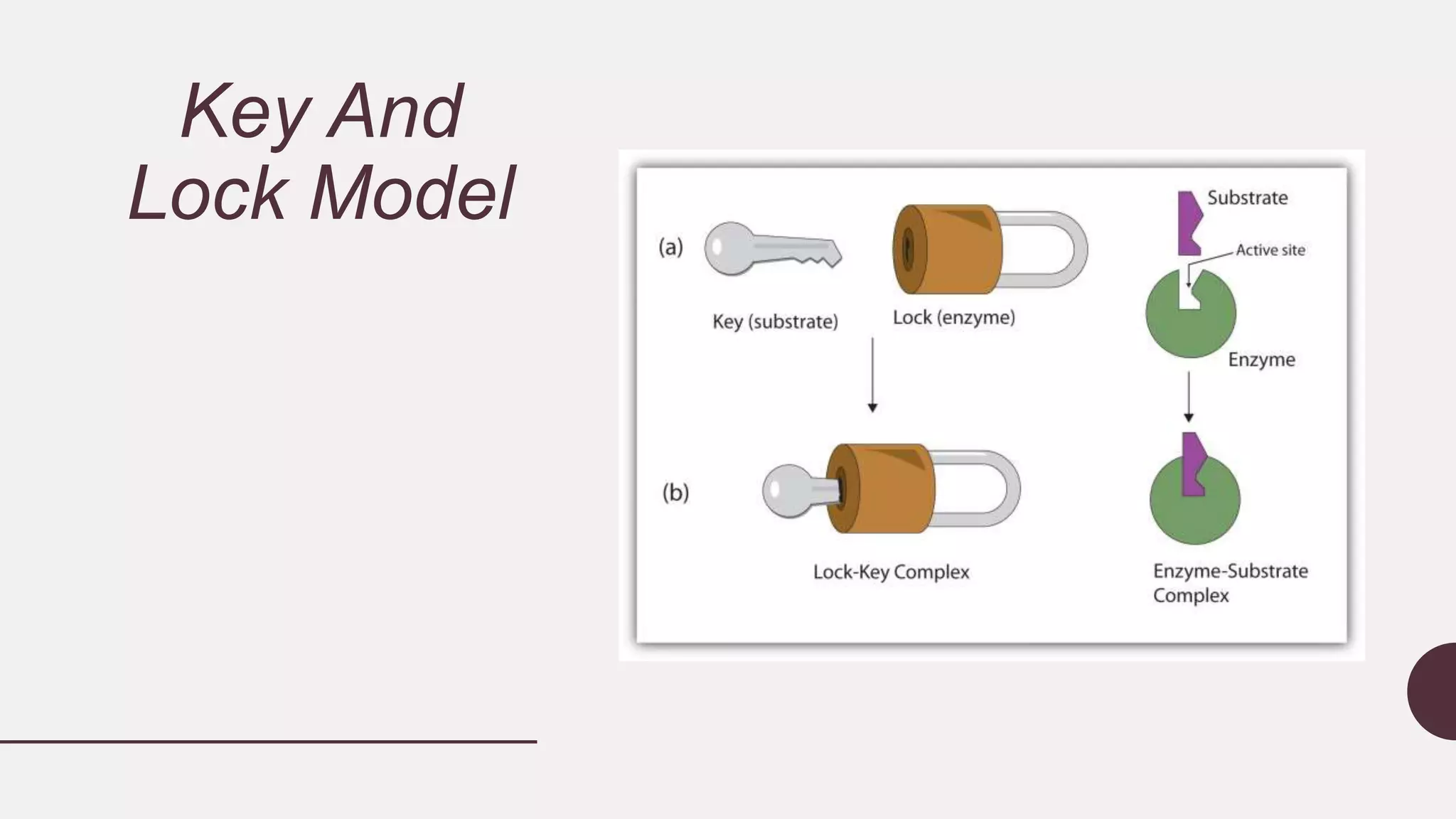
This document discusses enzyme kinetics and provides details about key concepts. It introduces enzymes and their role in catalyzing reactions. It then describes enzyme kinetics as the study of reaction rates of enzyme-catalyzed reactions under varying conditions. Several key aspects of enzyme kinetics are summarized, including parametric analysis of enzyme catalysis, enzyme specificity, the lock and key model, and the Michaelis-Menten kinetics model. This model describes how reaction rates increase with substrate concentration until reaching a maximum rate Vmax as the enzyme becomes saturated.


![Michaelis–
Menten
kinetics
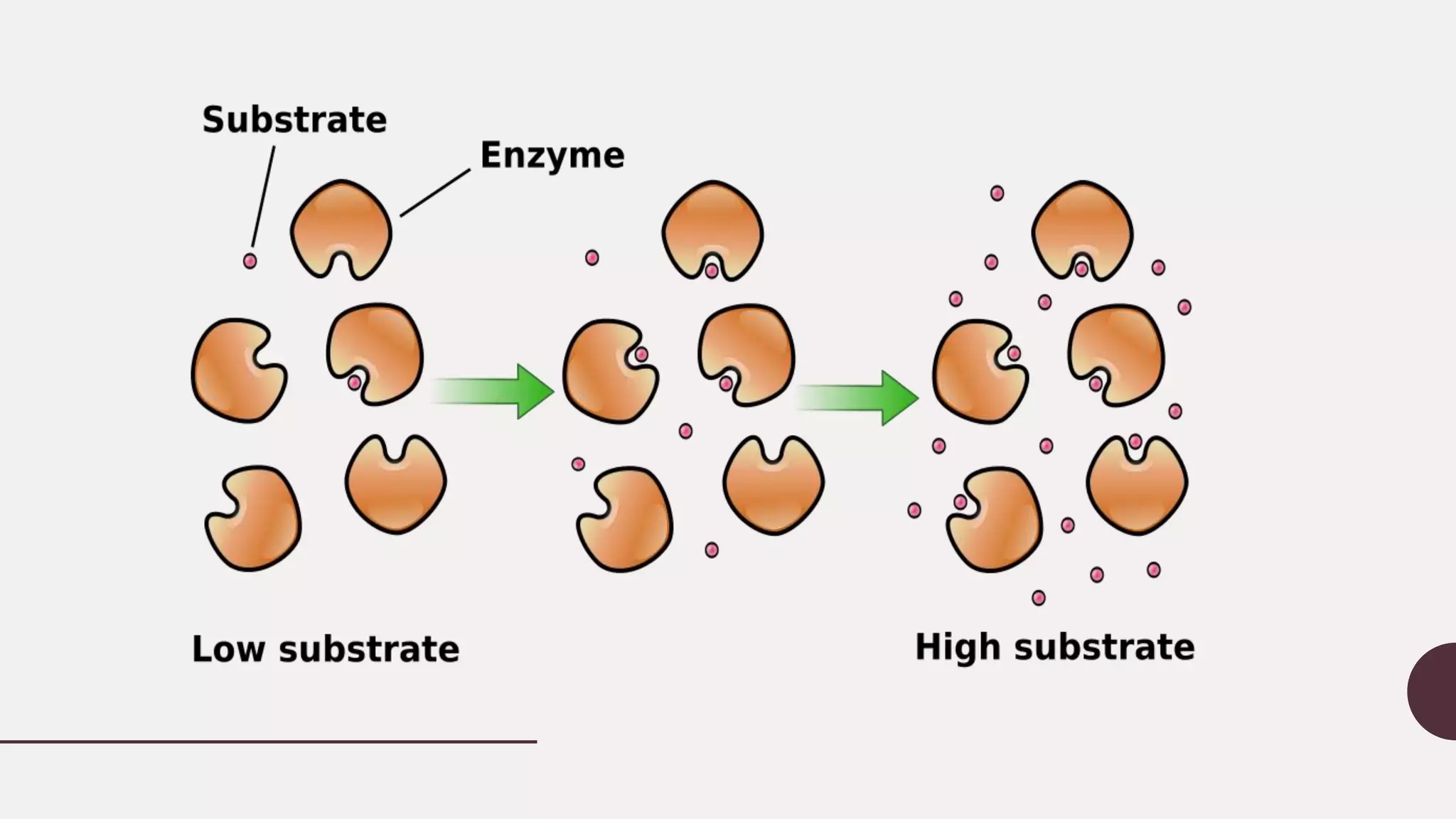
• As enzyme-catalyzed reactions are
saturable, their rate of catalysis does
not show a linear response to
increasing substrate. If the initial
rate of the reaction is measured over
a range of substrate concentrations
(denoted as [S]), the reaction rate (v)
increases as [S] increases, as shown
on the right. However, as [S] gets
higher, the enzyme becomes
saturated with substrate and the
rate reaches Vmax, the enzyme's
maximum rate.](https://image.slidesharecdn.com/enzymekineticspresentation-160521155214/75/Enzyme-kinetics-presentation-12-2048.jpg)