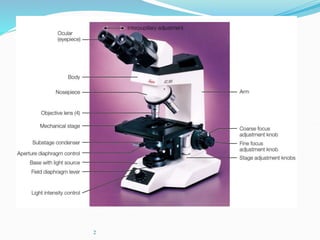
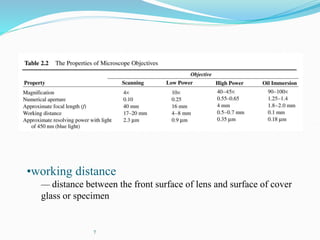
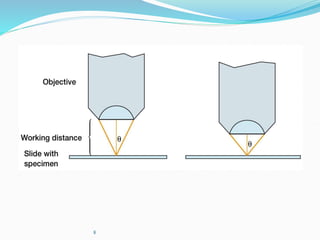
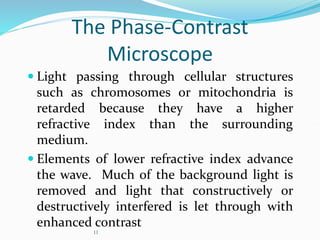
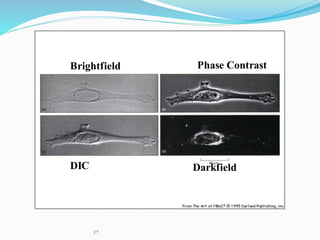
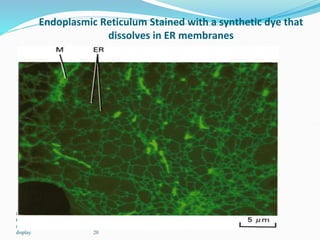
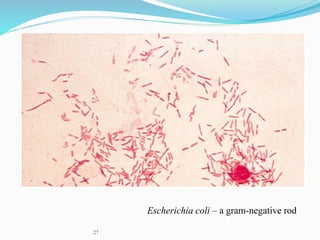
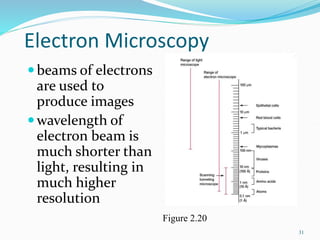
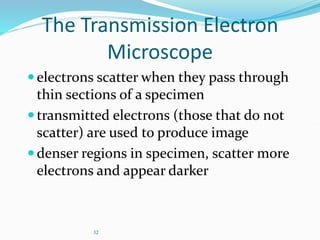
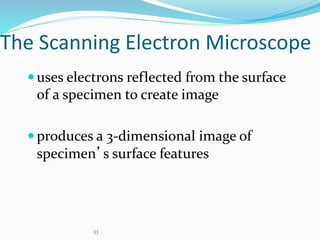
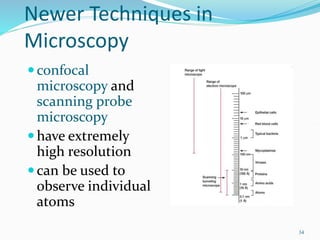
The document discusses different types of light microscopes and their uses. It describes brightfield, darkfield, phase contrast, fluorescence, confocal and electron microscopes. Brightfield microscopes produce a dark image on a bright background using transmitted light. Phase contrast and darkfield microscopes enhance contrast in unstained samples. Fluorescence microscopes use fluorescent dyes and emitted light. Confocal and electron microscopes provide higher resolution images using lasers or electron beams respectively. Specimen preparation through fixing, staining and sectioning is also outlined.