There are several types of chemical reactions:
1) Combination reactions involve two or more substances combining to form a new compound such as reactions of elements with oxygen or metals with halogens.
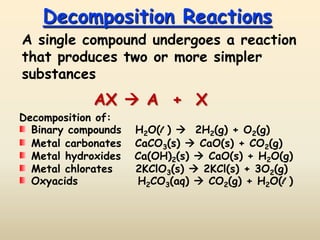
2) Decomposition reactions involve a single compound breaking down into simpler substances like the decomposition of water into hydrogen and oxygen gases.
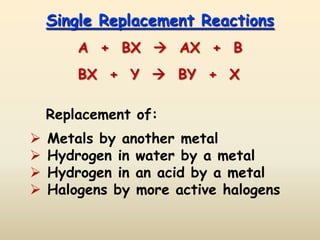
3) Single replacement reactions involve a reaction where one element replaces another in a compound such as a metal replacing hydrogen in an acid.
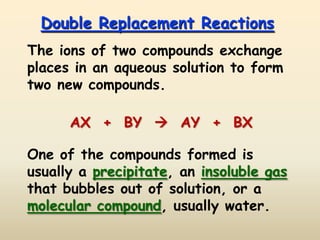
4) Double replacement reactions involve the ions of two compounds exchanging places to form two new compounds, often with a precipitate forming.