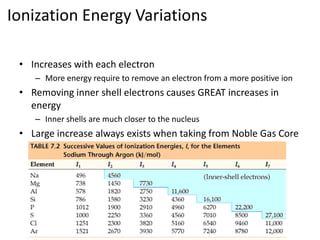
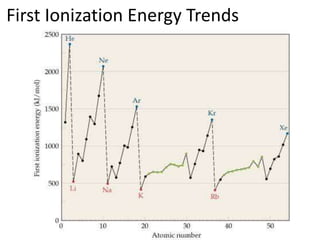
The document discusses ionization energy, which is the minimum energy required to remove an electron from an isolated gaseous atom or ion. It notes that ionization energy increases with each electron removed, and there is a large increase when removing inner shell electrons closer to the nucleus. Ionization energy also follows periodic trends, increasing within rows of the periodic table and decreasing within columns, with smaller atoms tending to have higher ionization energies due to being closer to the nucleus. Electrons are always removed from the highest principal energy level when forming ions.










![Answers
• (D) 1s2 2s2 2p6 3s1
• Metals
• [Ar]3d6
• K<Na<P<Ar<Ne
• True](https://image.slidesharecdn.com/chempowerpoint-121104134258-phpapp03-121104180341-phpapp01/85/Chempowerpoint-121104134258-phpapp03-13-320.jpg)
