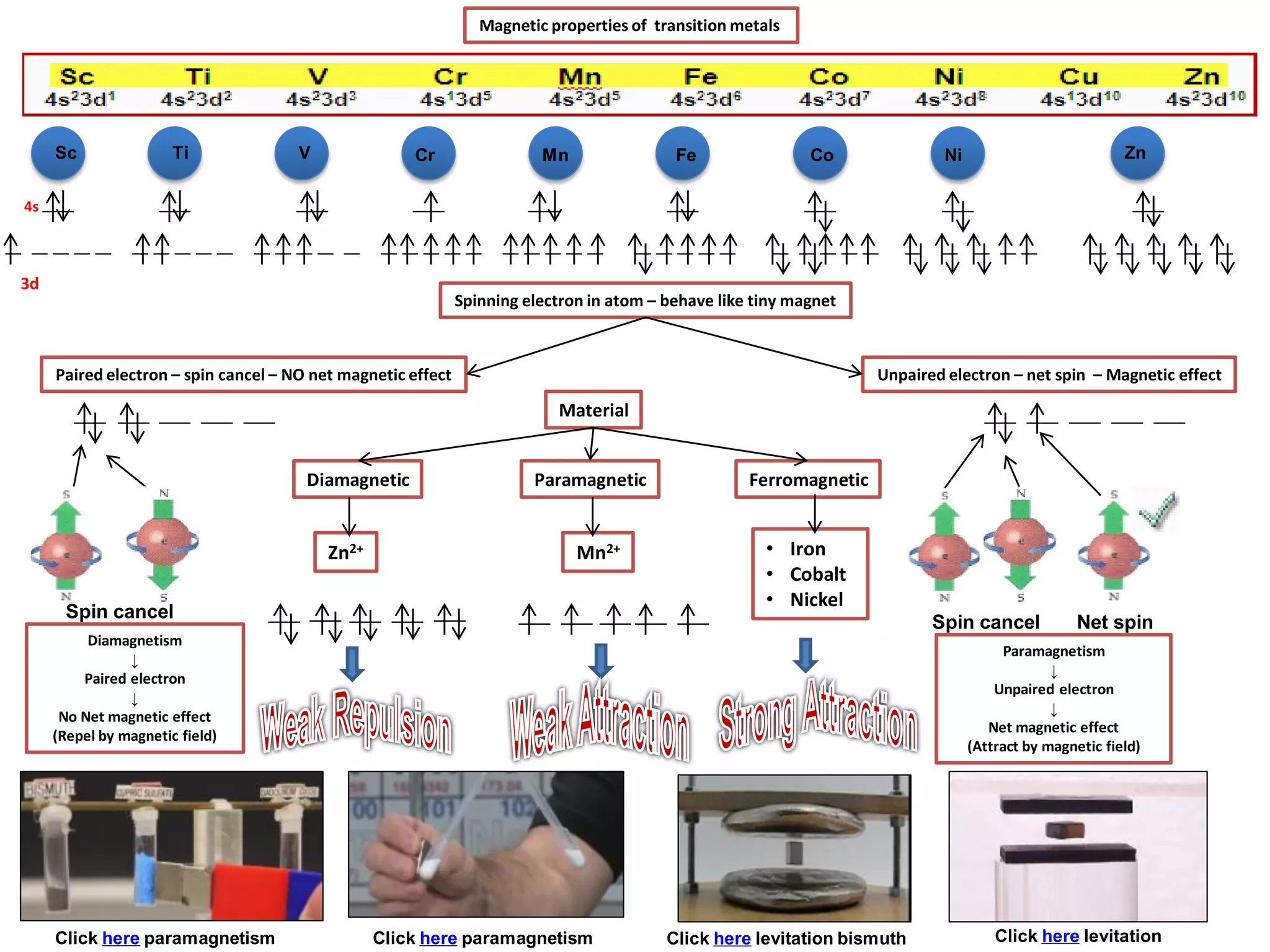
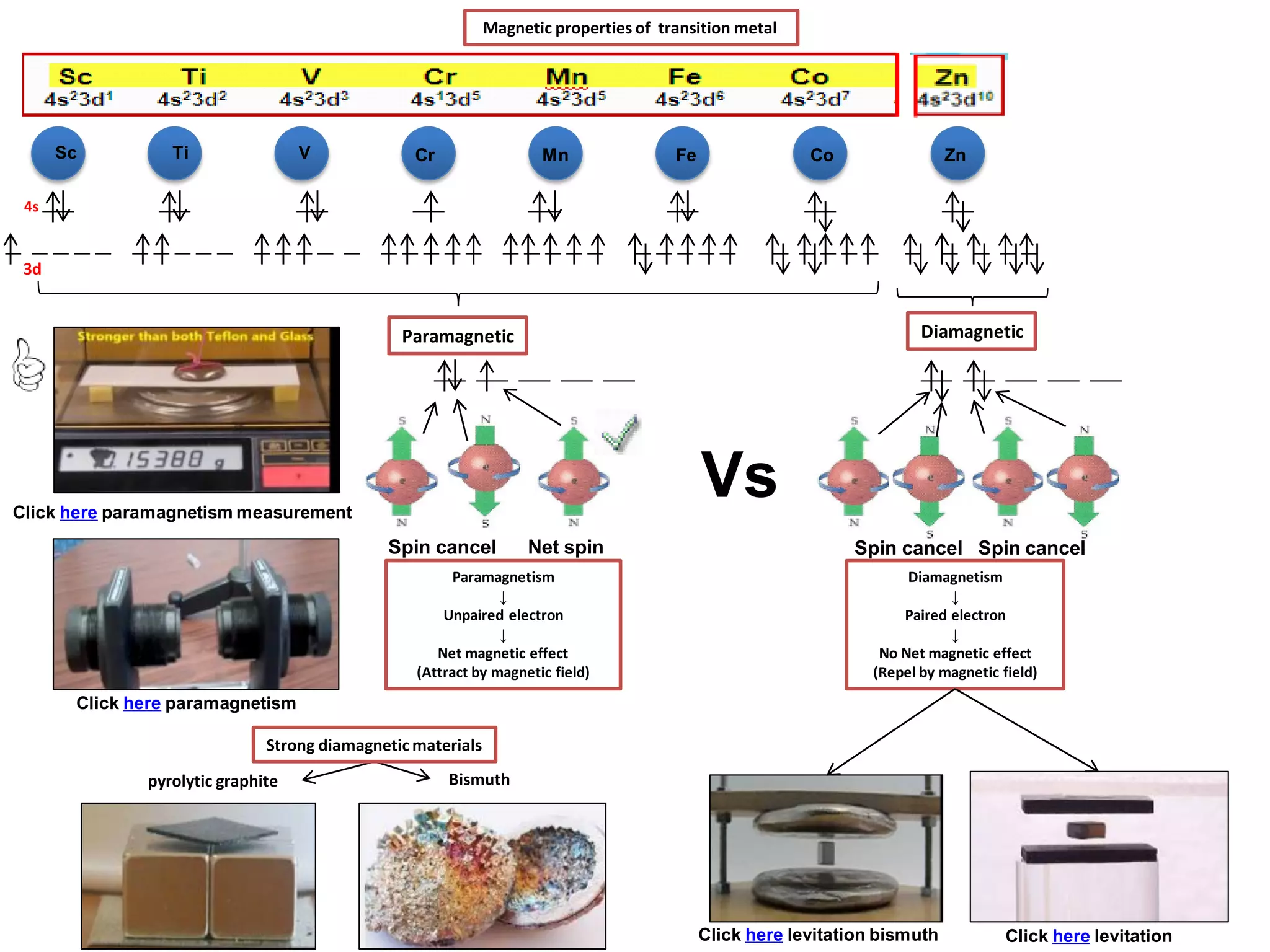
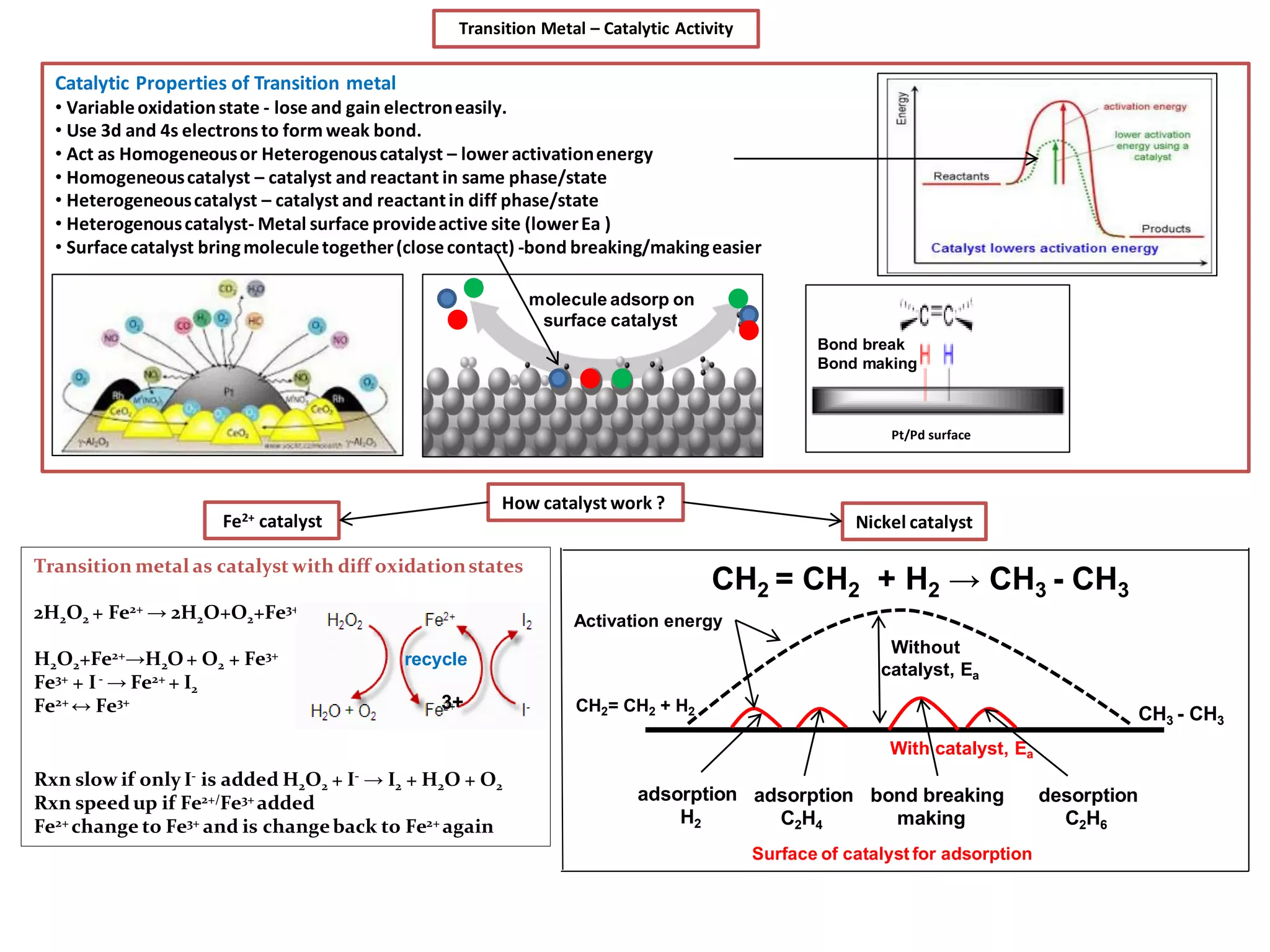
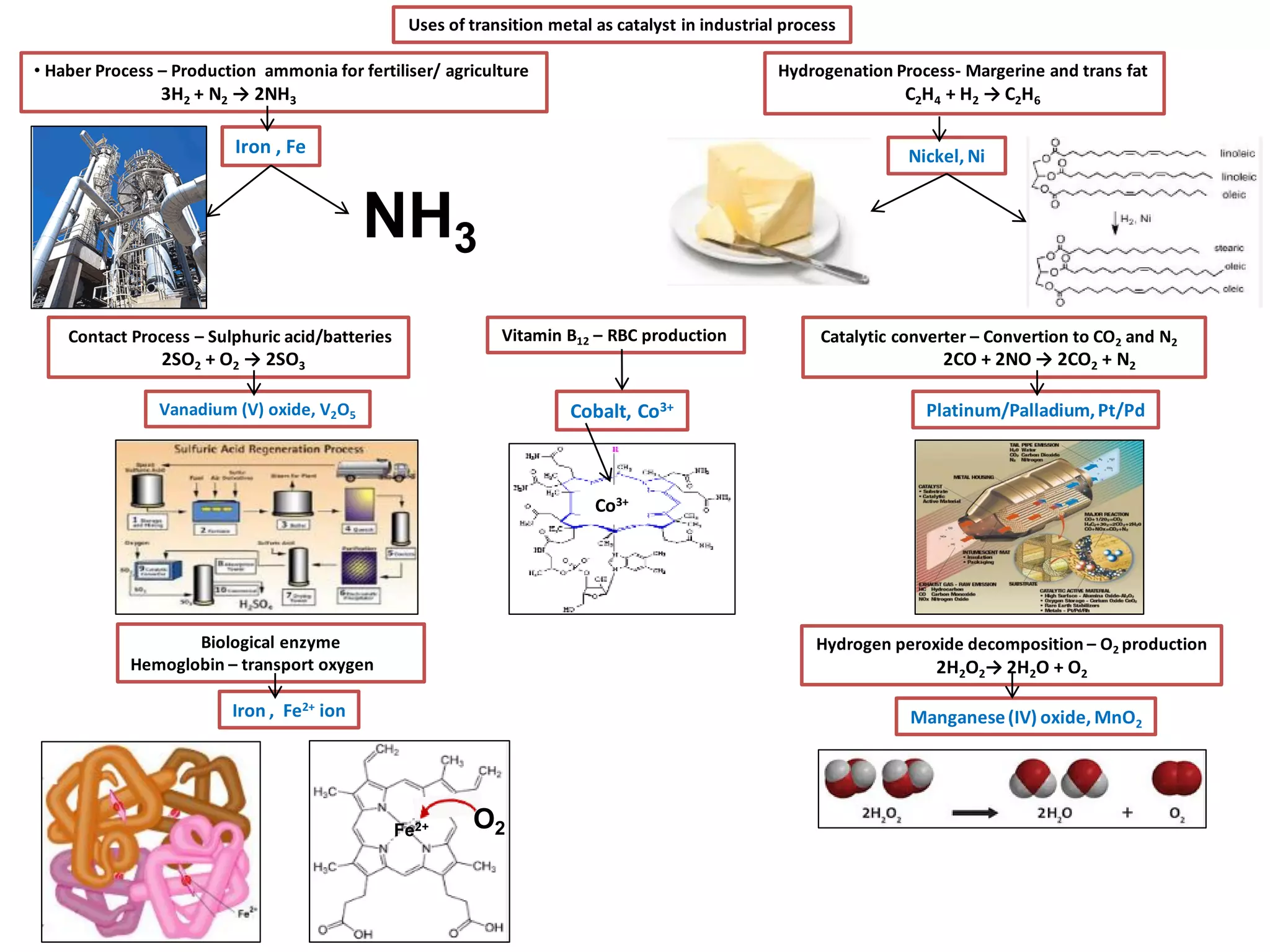
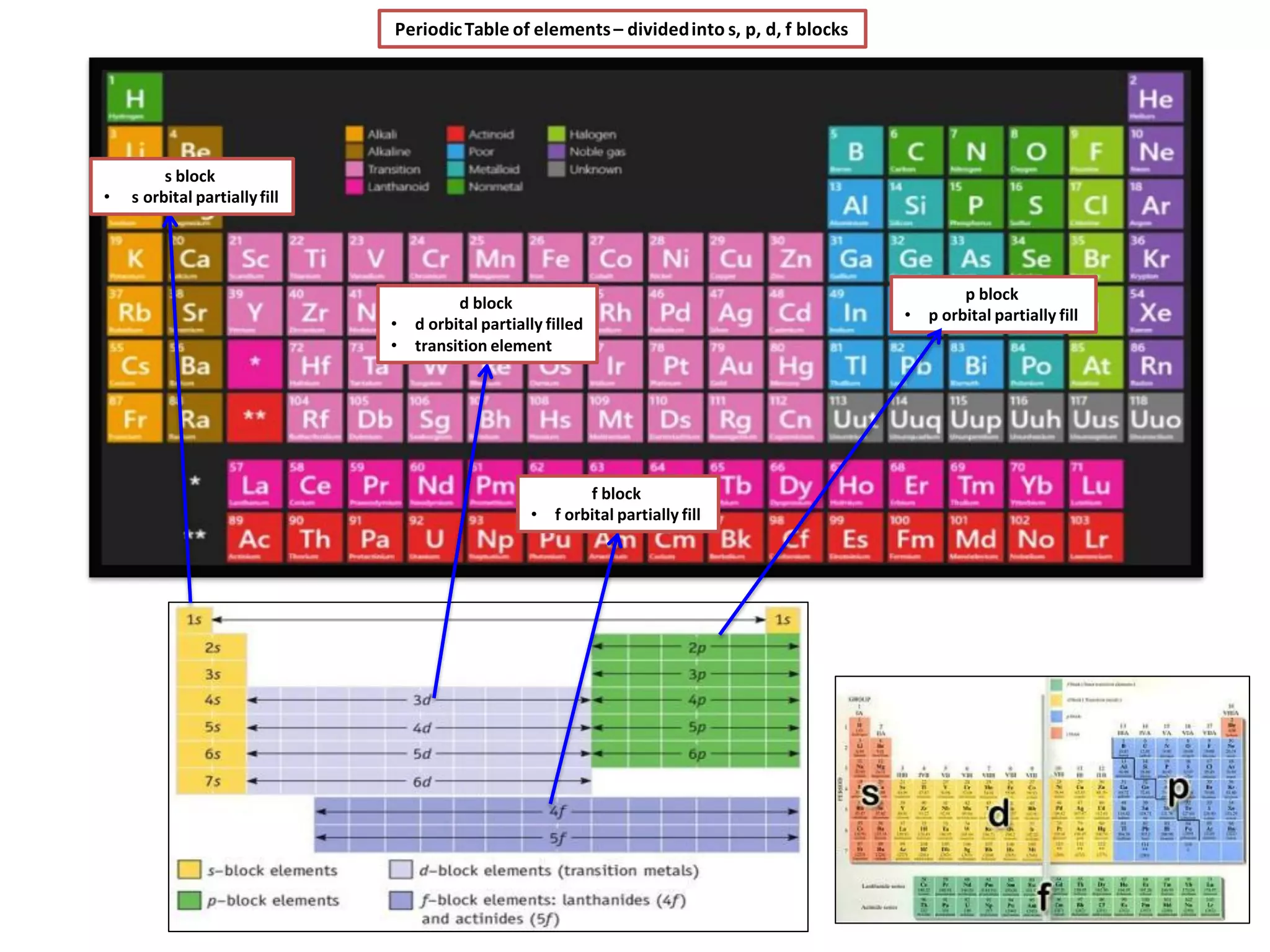
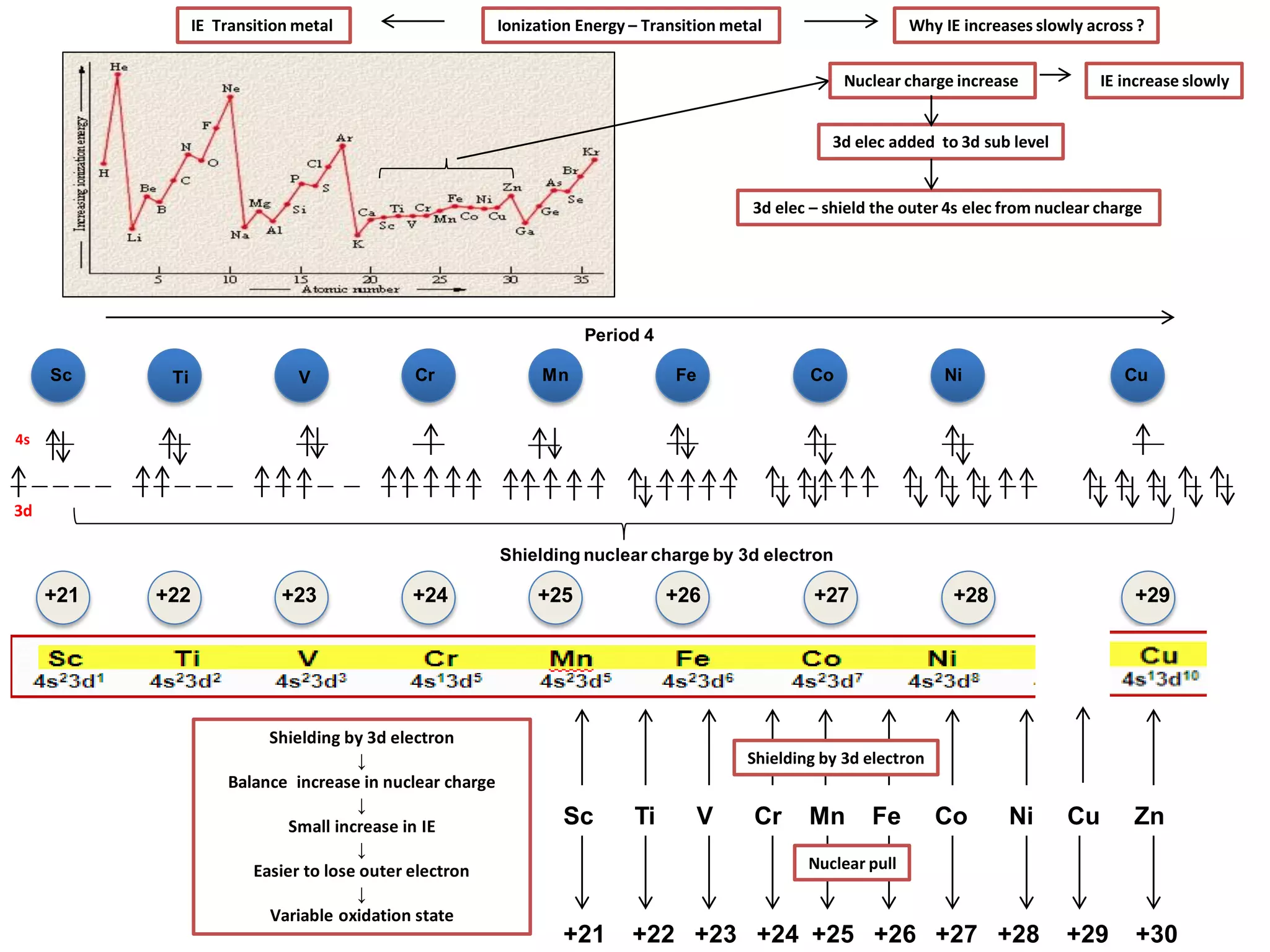
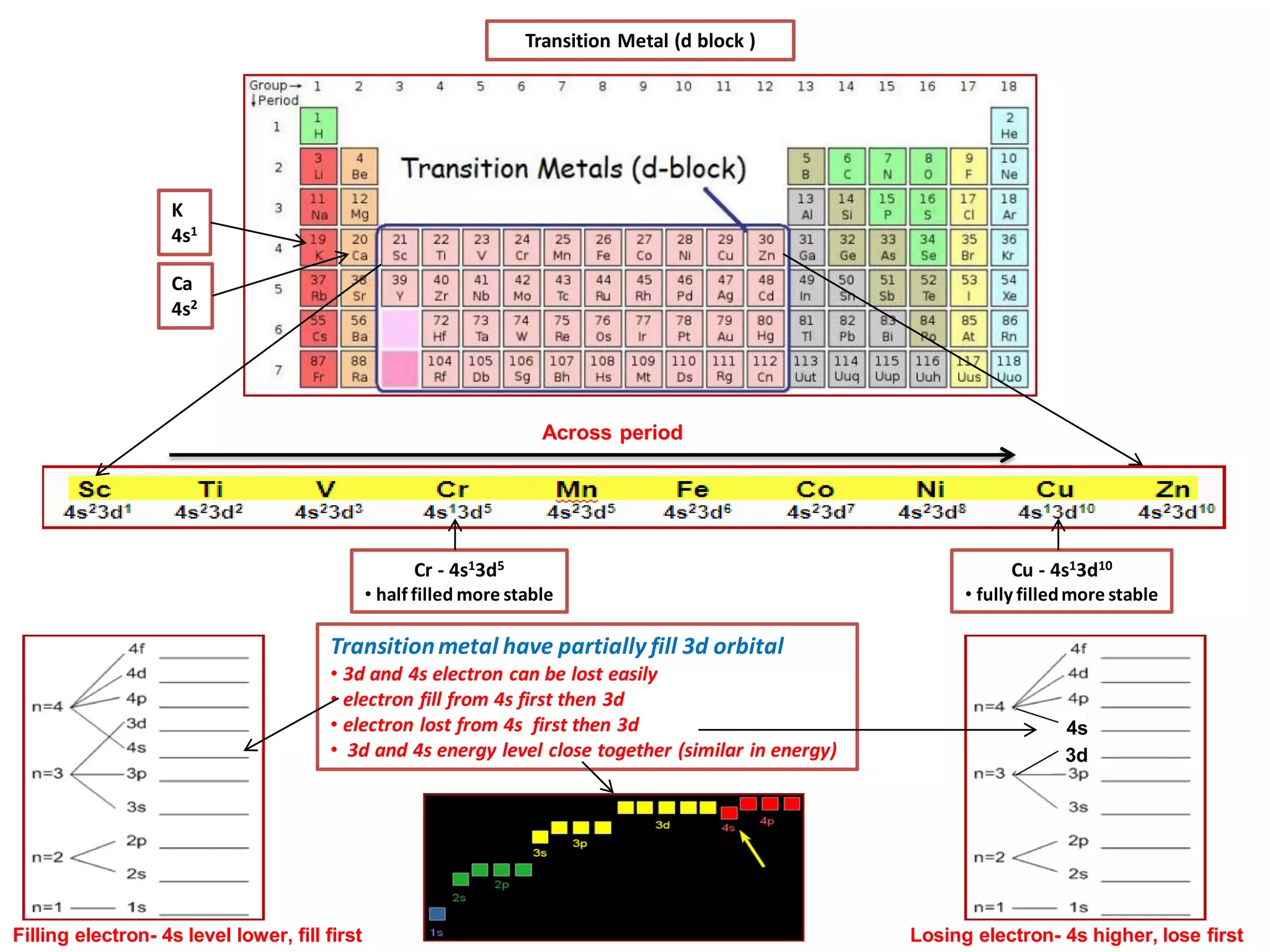
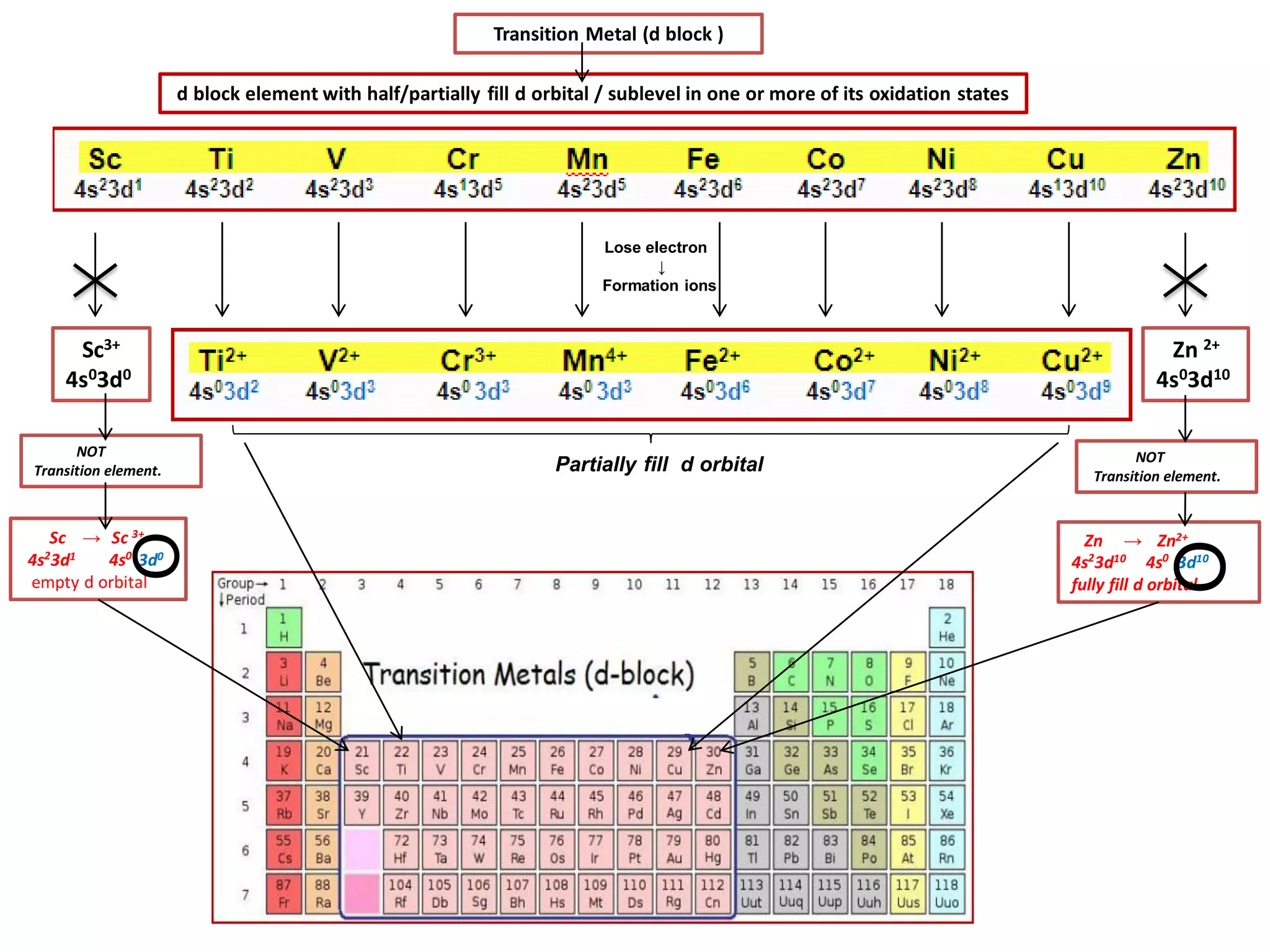
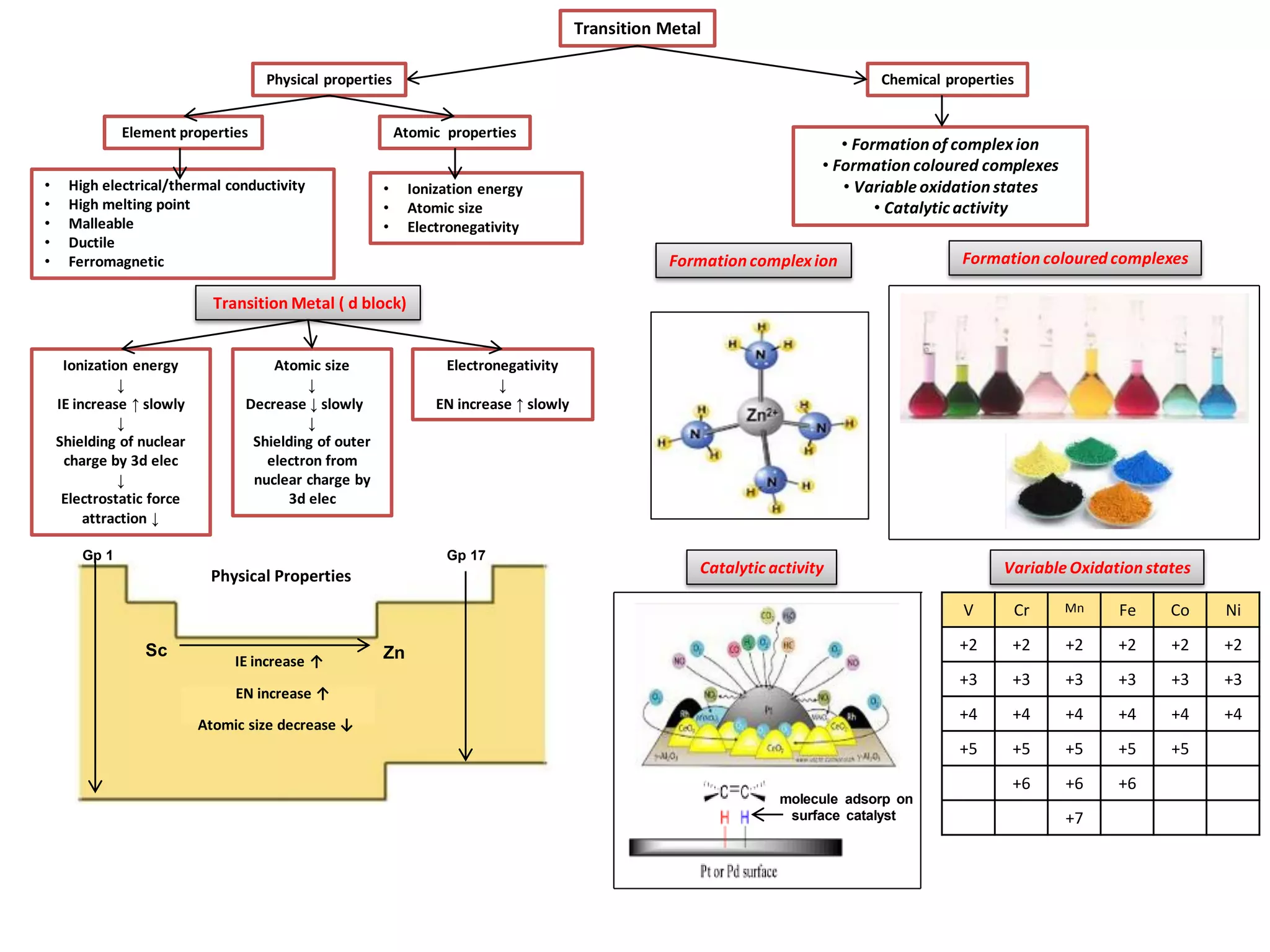
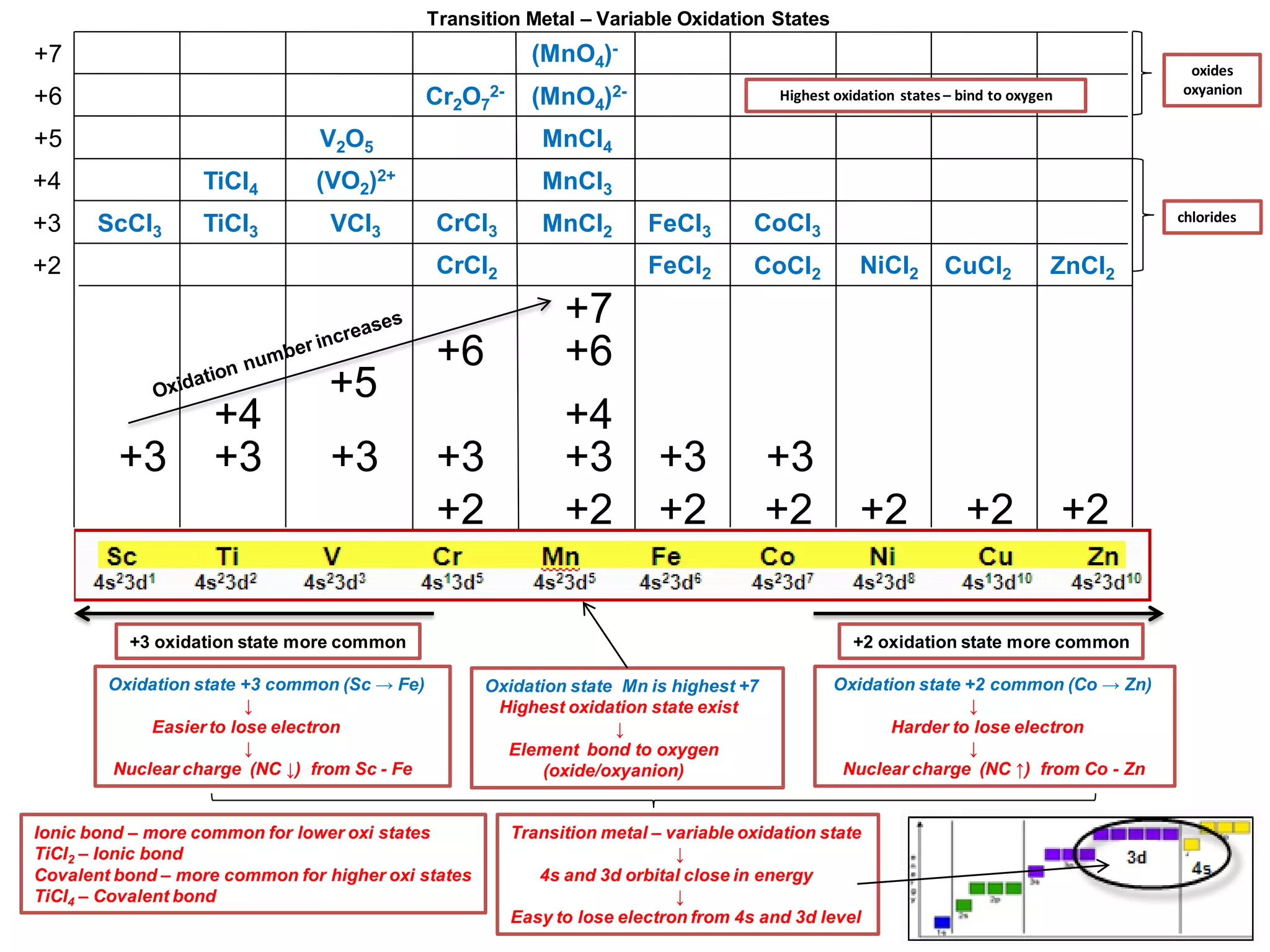
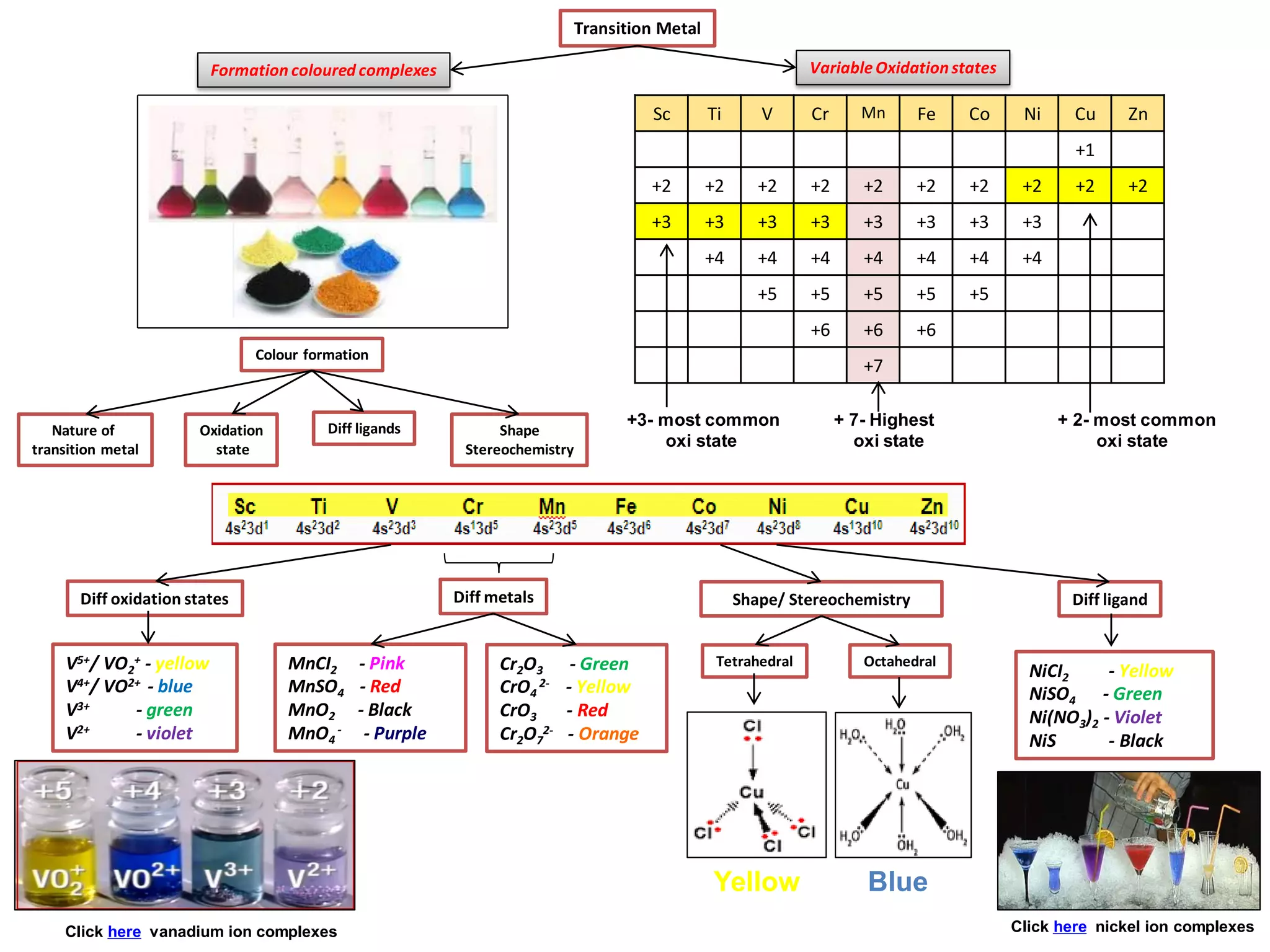
The document discusses the periodic table and properties of elements. It is divided into blocks based on orbital filling: s, p, d, and f blocks. Transition metals are in the d block and have partially filled d orbitals. They exhibit variable oxidation states, can form colored complexes, and show catalytic activity due to this electronic configuration. Magnetic properties depend on paired or unpaired electrons in the outer shell.
![Periodic Table – s, p d, f block elements block elements
• s orbitals partially fill
p block elements
• p orbital partially fill
d block elements
• d orbitals partially fill
• transition elements
1 H 1s1
2 He 1s2
11 Na [Ne] 3s1
12 Mg [Ne] 3s2
5 B [He] 2s2 2p1
6 C [He] 2s2 2p2
7 N [He] 2s2 2p3
8 O [He] 2s2 2p4
9 F [He] 2s2 2p5
10 Ne [He] 2s2 2p6
13 Al [Ne] 3s2 3p1
14 Si [Ne] 3s2 3p2
15 P [Ne] 3s2 3p3
16 S [Ne] 3s2 3p4
17 CI [Ne] 3s2 3p5
18 Ar [Ne] 3s2 3p6
19 K [Ar] 4s1
20 Ca [Ar] 4s2
21 Sc [Ar] 4s2 3d1
22 Ti [Ar] 4s2 3d2
23 V [Ar] 4s2 3d3
24 Cr [Ar] 4s1 3d5
25 Mn [Ar] 4s2 3d5
26 Fe [Ar] 4s2 3d6
27 Co [Ar] 4s2 3d7
28 Ni [Ar] 4s2 3d8
29 Cu [Ar] 4s1 3d10
30 Zn [Ar] 4s2 3d10
n = 2 period 2
3 Li [He] 2s1
4 Be [He] 2s2
Click here video s,p,d,f blocks,Click here video on s,p,d,f notationClick here electron structure
Video on electron configuration
f block elements
• f orbitals partially fill](https://image.slidesharecdn.com/sfcwi9hmqb2zfgrskfms-signature-bb3f64ce4fabf0f8a44248b6a84898ed7030e768089e0dd026da563ec80894ac-poli-150620043145-lva1-app6891/75/IB-Chemistry-on-Properties-of-Transition-Metal-and-Magnetism-2-2048.jpg)
![Periodic Table – s, p d, f blocks element
Electron structure
Chromium d block (Period 4)
1s2 2s2 2p6 3s2 3p6 4s1 3d5
[Ar] 4s1 3d5
Electron structure
Germanium p block, Gp 14 (Period 4)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
[Ar] 4s2 3d10 4p2
Electron structure
Lead p block, Gp 14 (Period 6)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10 5p6 6s2 4f14 5d106p2
[Xe] 6s2 4f14 5d10 6p2
Electron structure
Iodine p block, Gp 7 (Period 5)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10 5p5
[Kr] 5s2 4d10 5p5
Electron structure
Cadmium d block (Period 5)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10
[Kr] 5s2 4d10
Electron structure
Mercury d block (Period 6)
1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s2 4d10 5p6 6s2 4f14 5d10
[Xe] 6s2 4f14 5d10
Gp 14 -4 valence electron Gp 17 - 7 valence electron
Gp 14 - 4 valence electrond block – d partially filledd block – d partially filled
d block – d partially filled
О
О
О
О
О
О](https://image.slidesharecdn.com/sfcwi9hmqb2zfgrskfms-signature-bb3f64ce4fabf0f8a44248b6a84898ed7030e768089e0dd026da563ec80894ac-poli-150620043145-lva1-app6891/75/IB-Chemistry-on-Properties-of-Transition-Metal-and-Magnetism-3-2048.jpg)
![Coordination
number
Shape Complex ion
(metal + ligand)
Ligand
(charged)
Metal ion
(Oxidation #)
Overall charge
on complex ion
linear [Cu(CI2)]- CI = -1 +1 - 1
[Ag(NH3)2]+ NH3 = 0 +1 + 1
[Ag(CN)2]- CN = -1 +1 - 1
Square
planar
[Cu(CI)4]2- CI = -1 +2 - 2
[Cu(NH3)4]2+ NH3 = 0 +2 +2
[Co(CI)4]2- CI = -1 +2 - 2
Tetrahedral [Cu(CI)4]2- CI = -1 +2 - 2
[Zn(NH3)4]2+ NH3 = 0 +2 + 2
[Mn(CI)4]2- CI = -1 +2 - 2
Octahedral [ Cu(H2O)6]2+ H2O = 0 +2 + 2
[Fe(OH)3(H2O)3] OH = -1
H2O = 0
+3 o
[Fe(CN)6]3- CN = -1 +3 - 3
[Cr(NH3)4CI2]+ NH3 = 0
CI = -1
+3 + 1
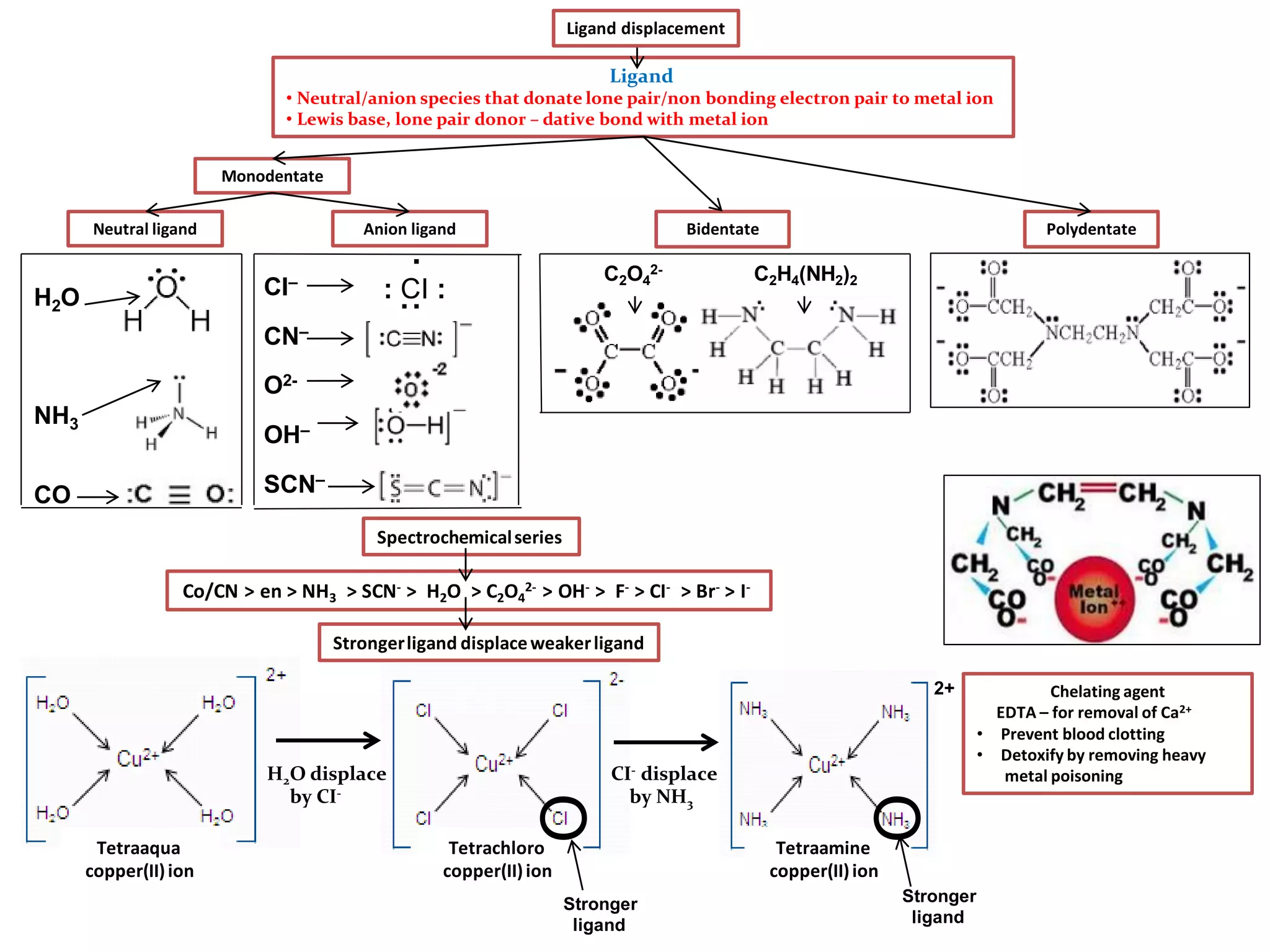
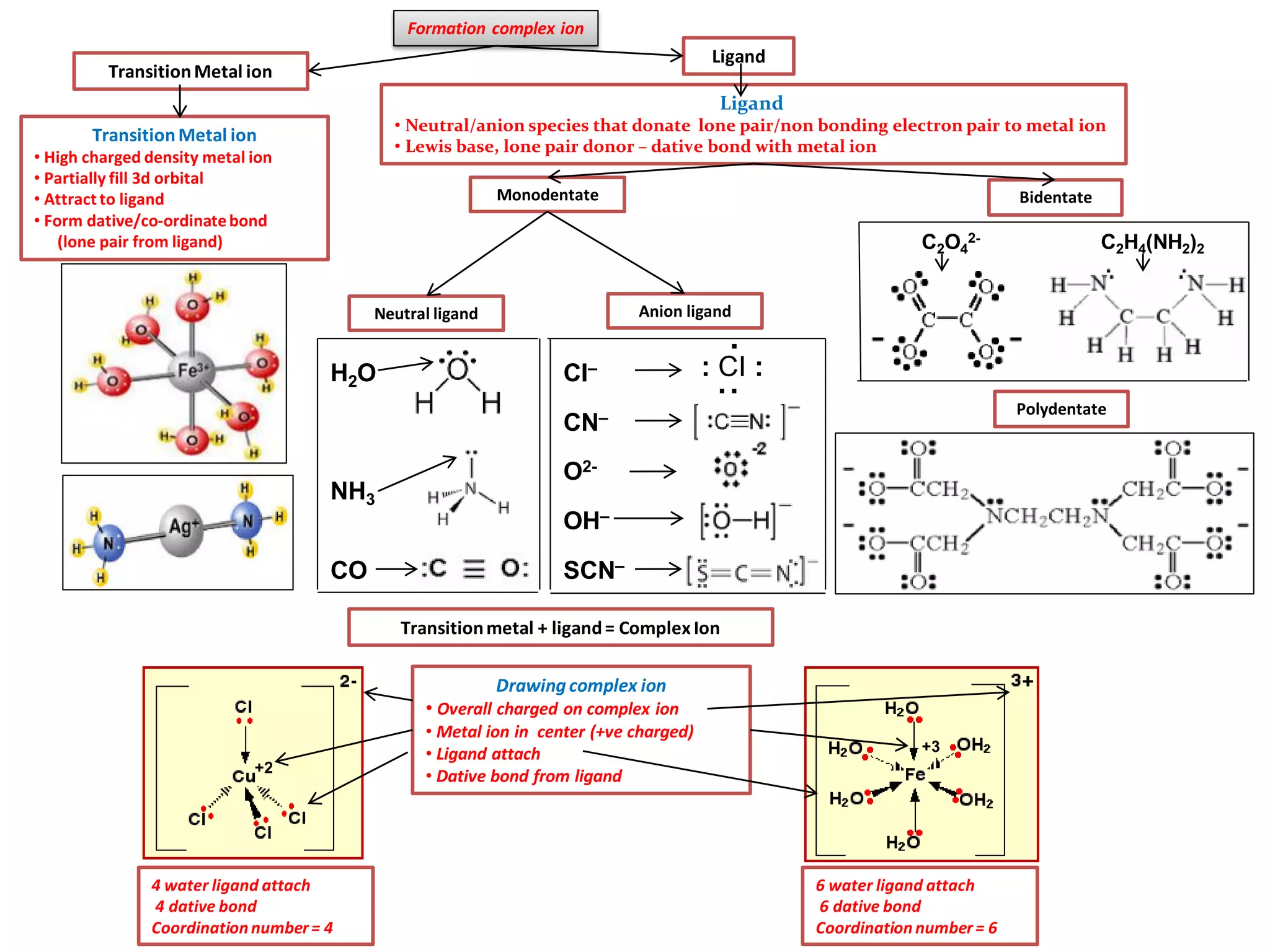
Types of ligand:
• Monodentate– 1 lone pair electrondonor– H2O, F-, CI-, NH3, OH-, SCN- CN-
• Bidentate – 2 lonepair electrondonor–1,2 diaminoethaneH2NCH2CH2NH2, ethanedioate(C2O4)2-
•Polydentate – 6 lone pair electrondonor– EDTA4- (ethylenediaminetetraaceticacid)
Complex ion with diff metal ion, ligand,oxidationstate and overallcharge
Mn+L: :L
Mn+
:L
:L
L:
L:
Mn+
:L
:L
:L
:L
Mn+
:L
:L
:L
:L
:L
:L
Coordination number
– number of ligand
around central ion
2
4
4
6](https://image.slidesharecdn.com/sfcwi9hmqb2zfgrskfms-signature-bb3f64ce4fabf0f8a44248b6a84898ed7030e768089e0dd026da563ec80894ac-poli-150620043145-lva1-app6891/75/IB-Chemistry-on-Properties-of-Transition-Metal-and-Magnetism-11-2048.jpg)