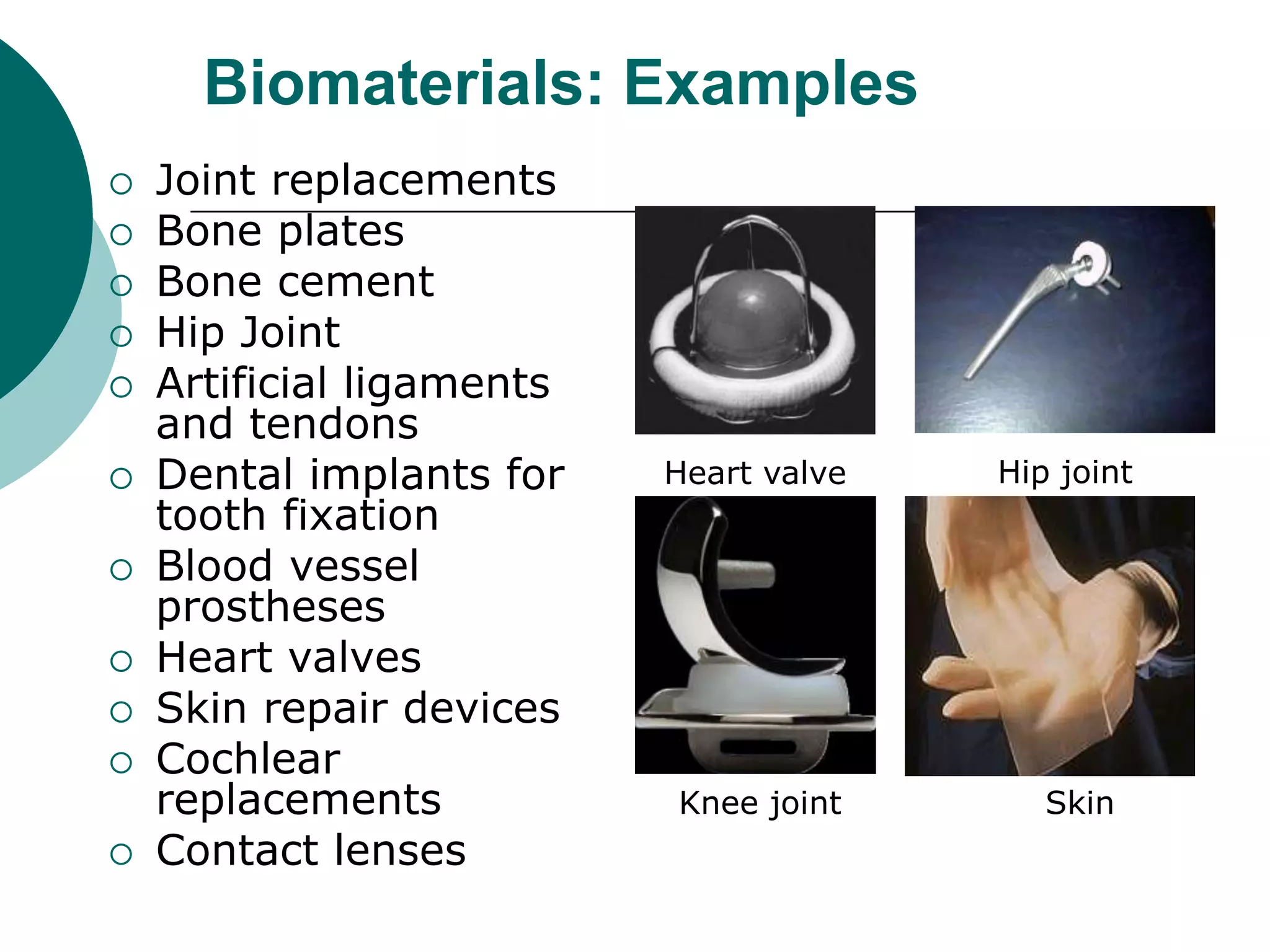
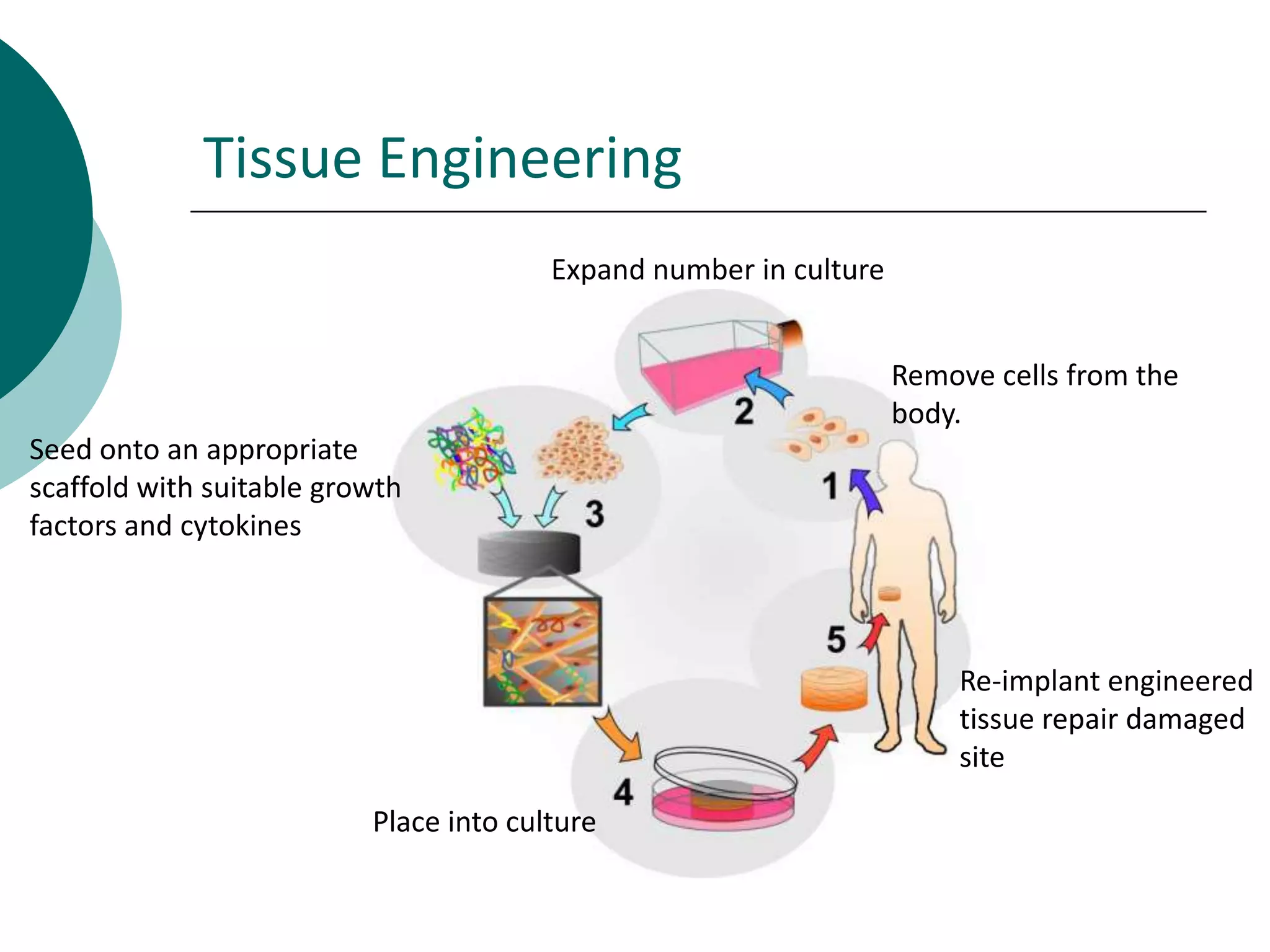
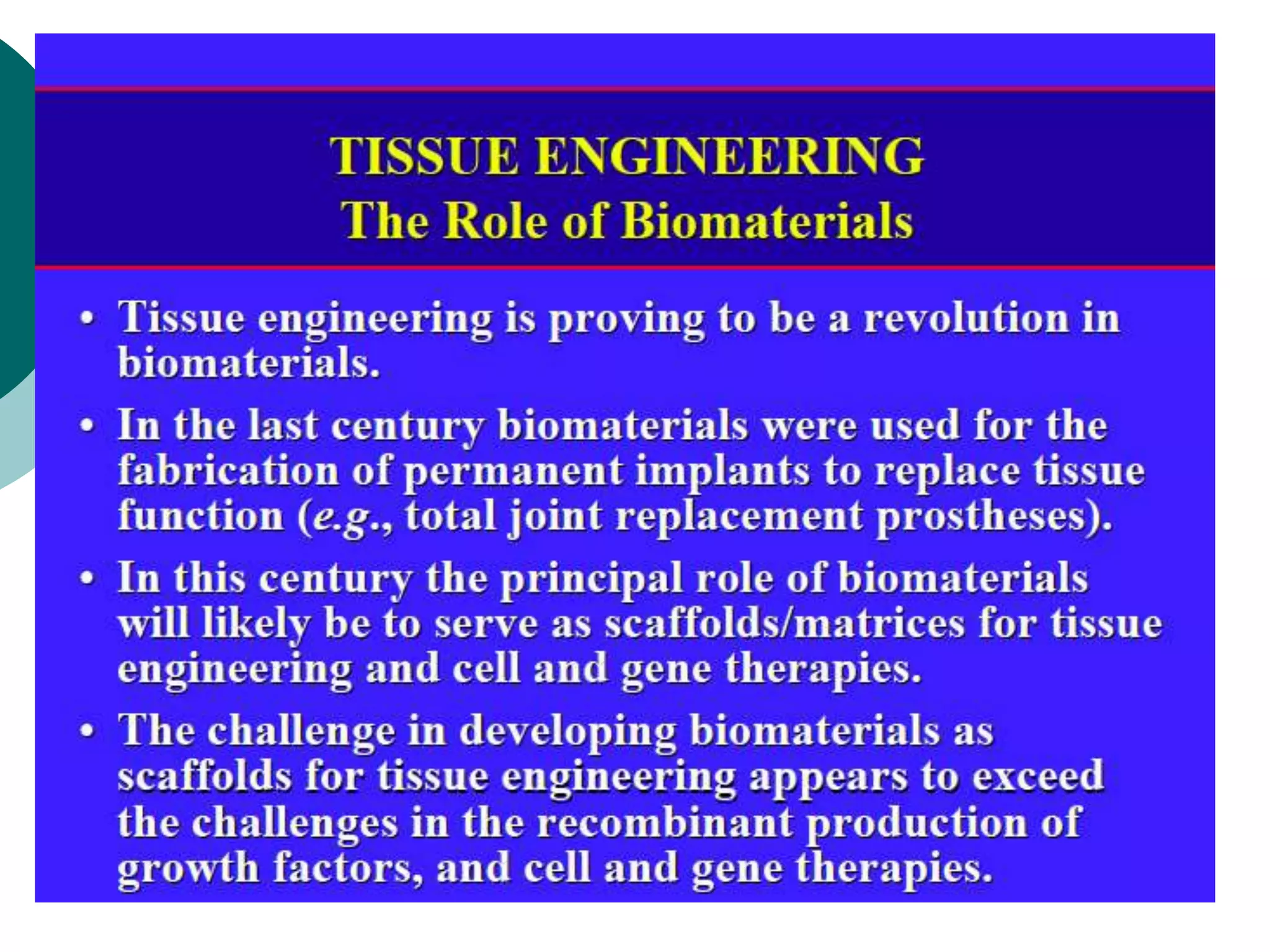
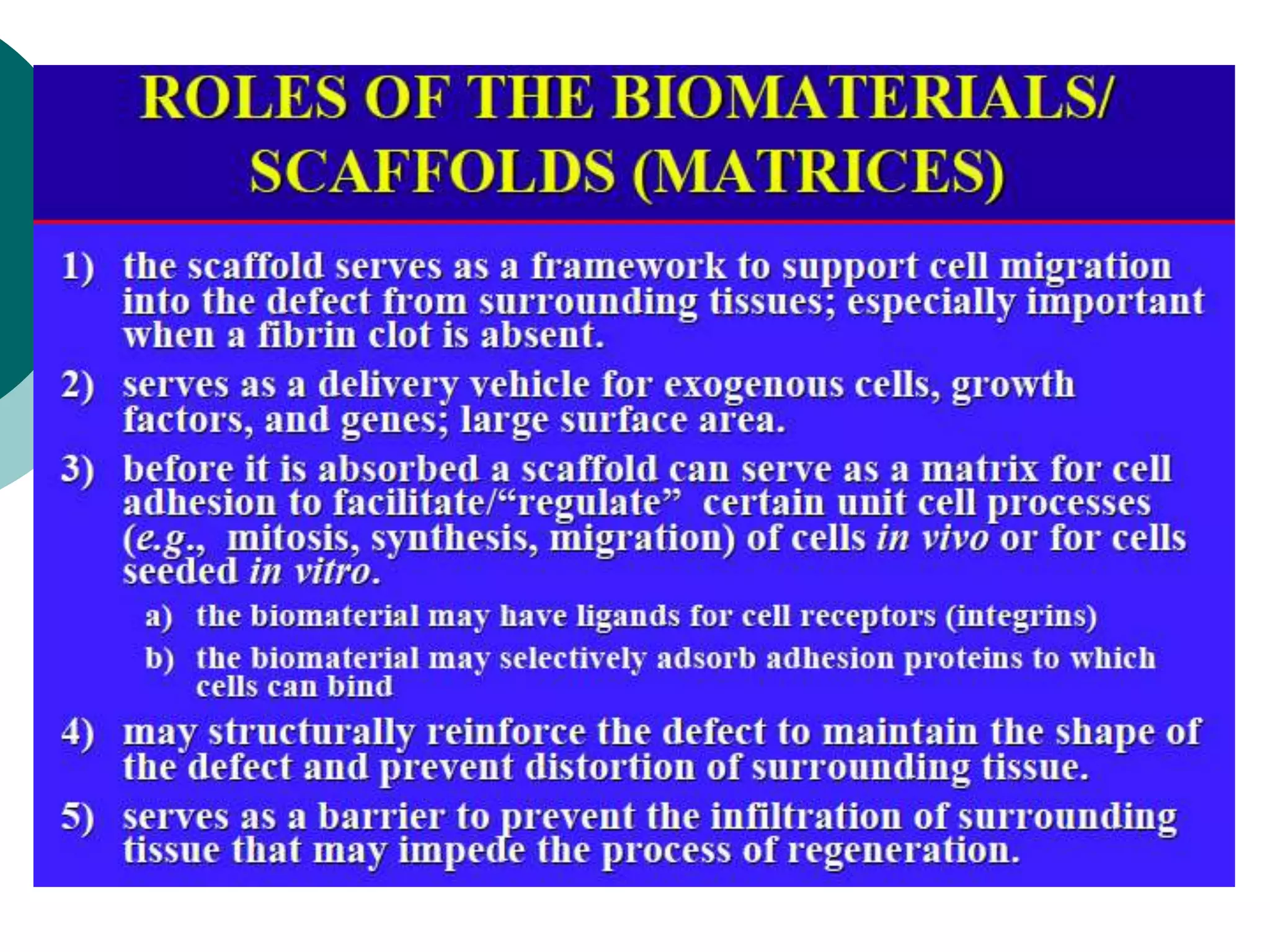
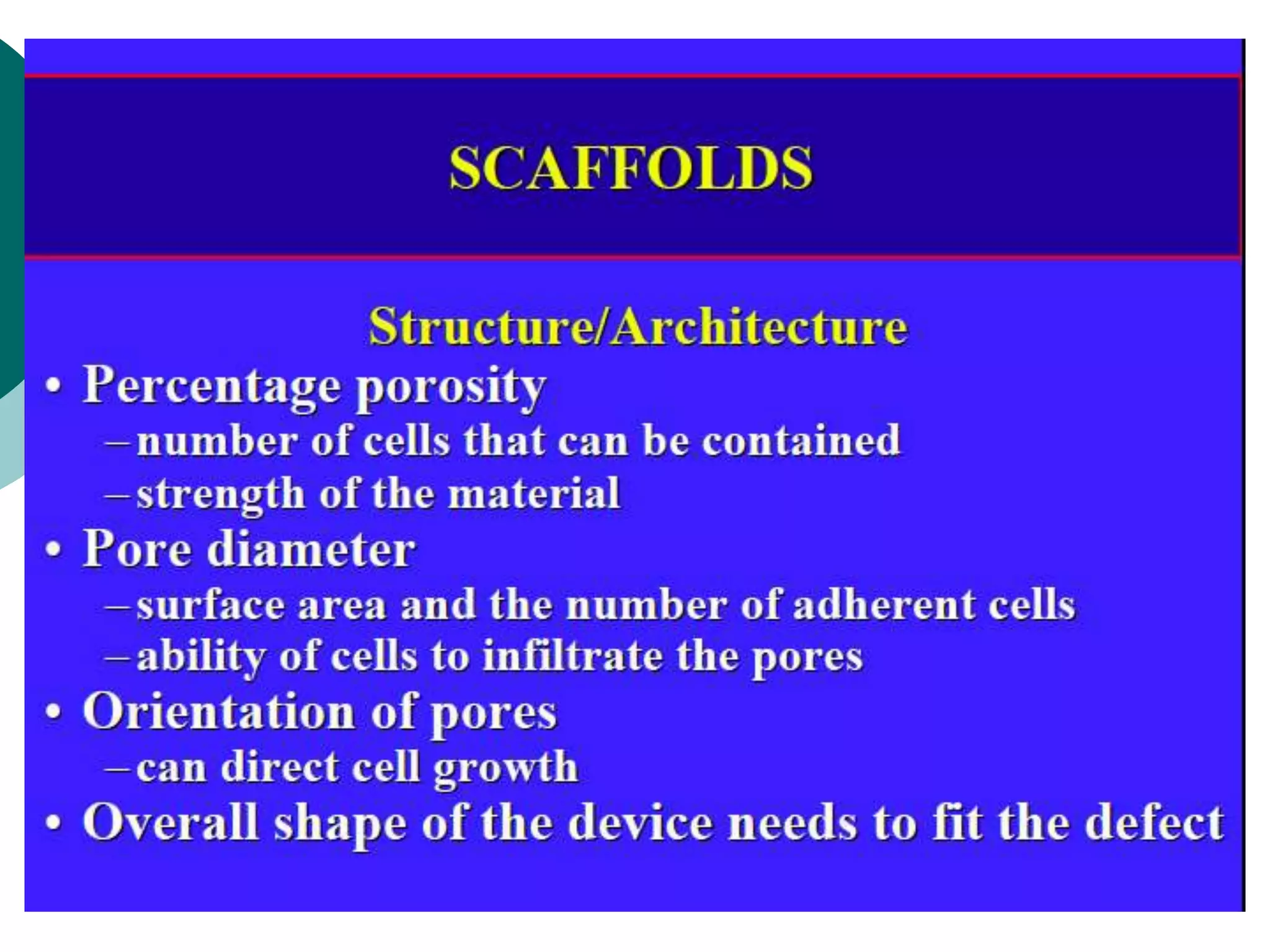
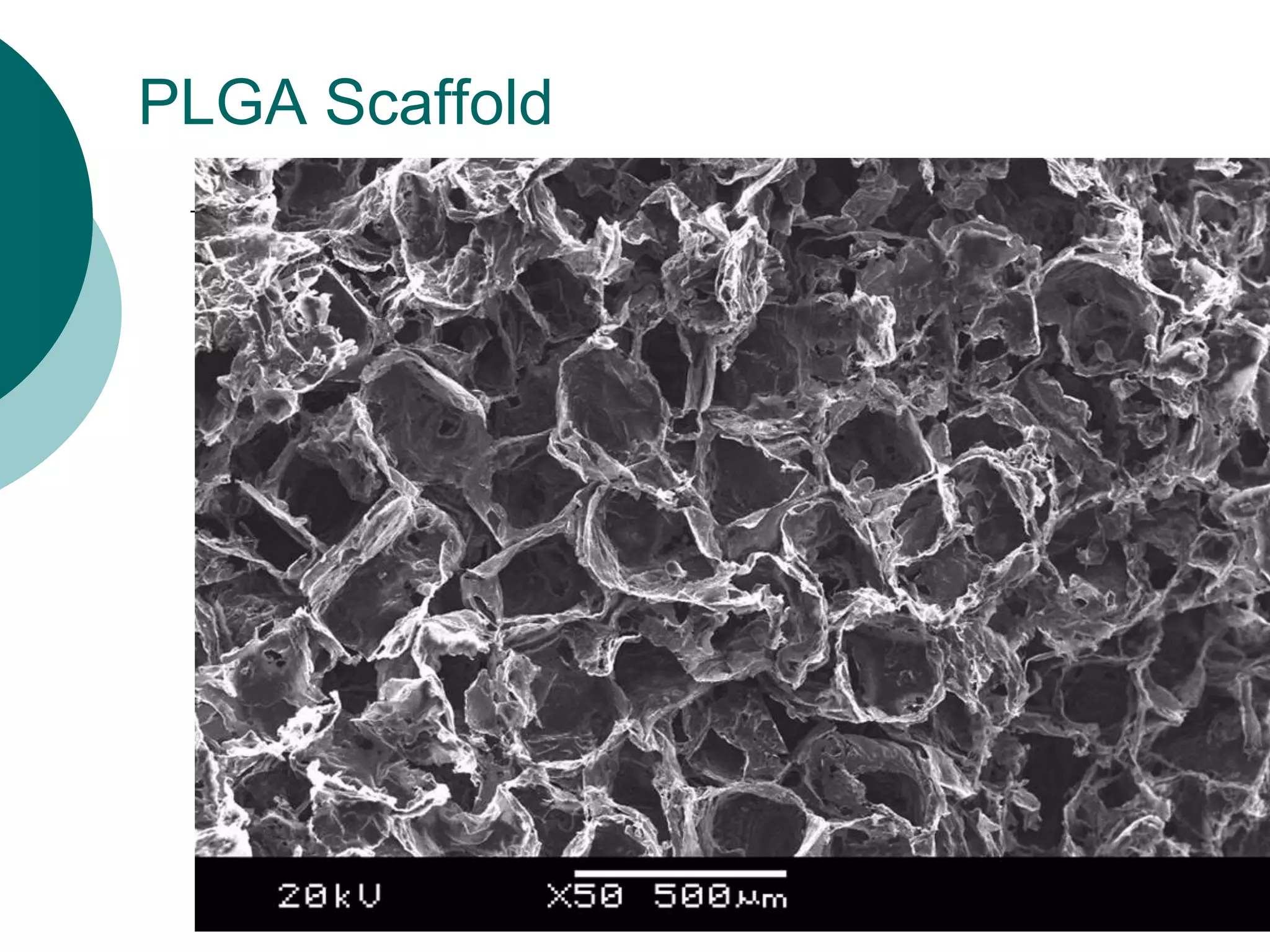
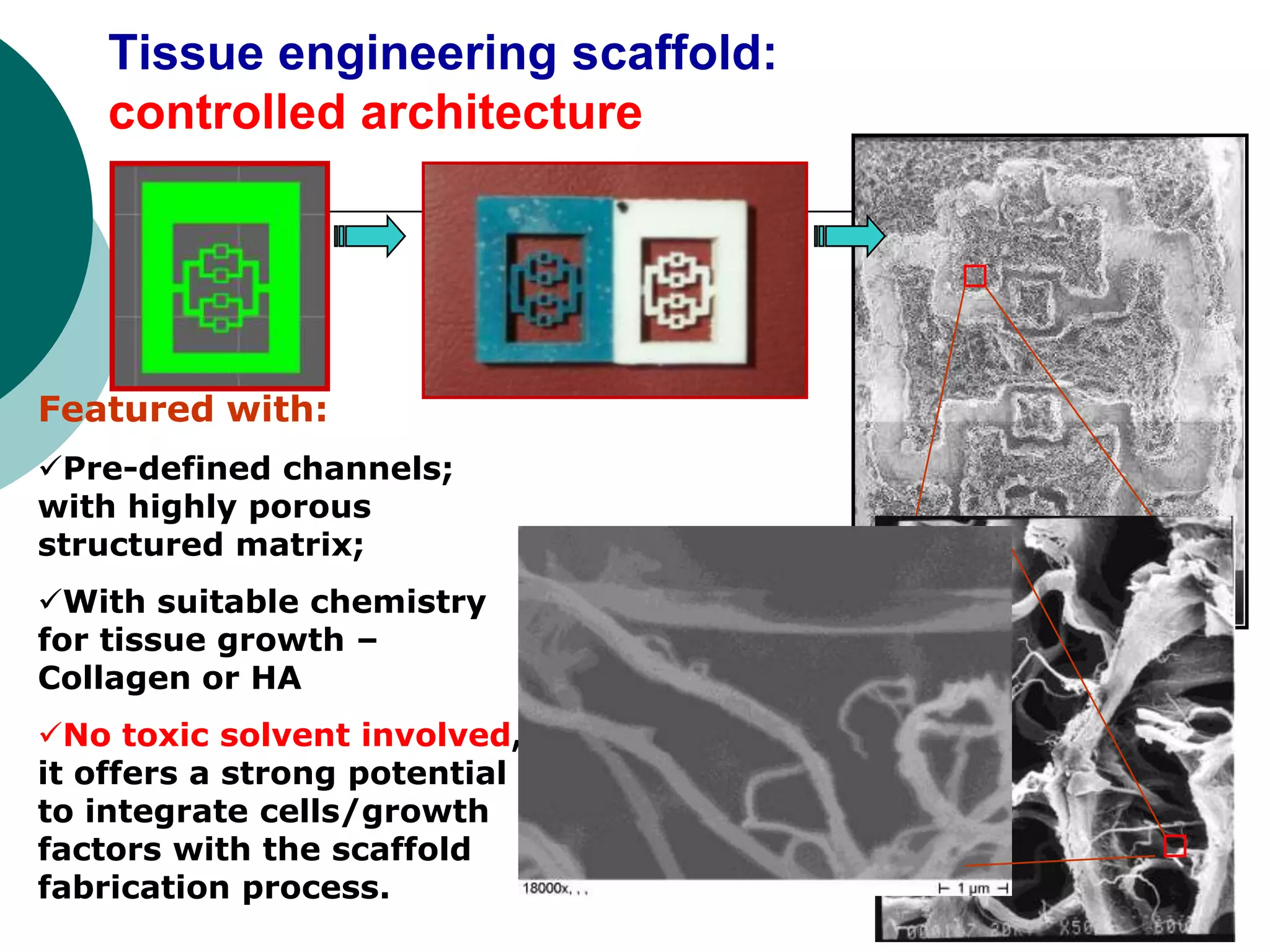
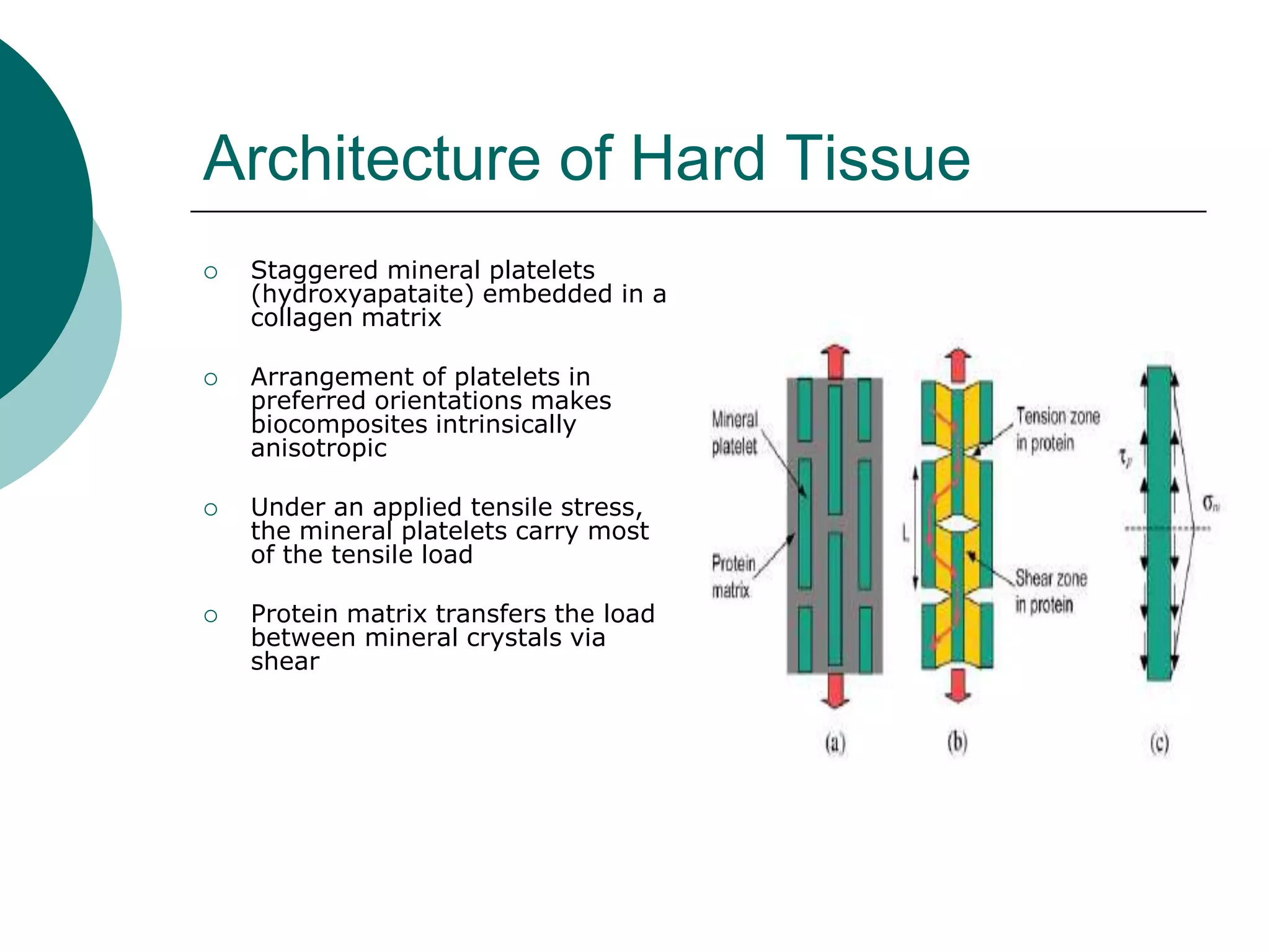
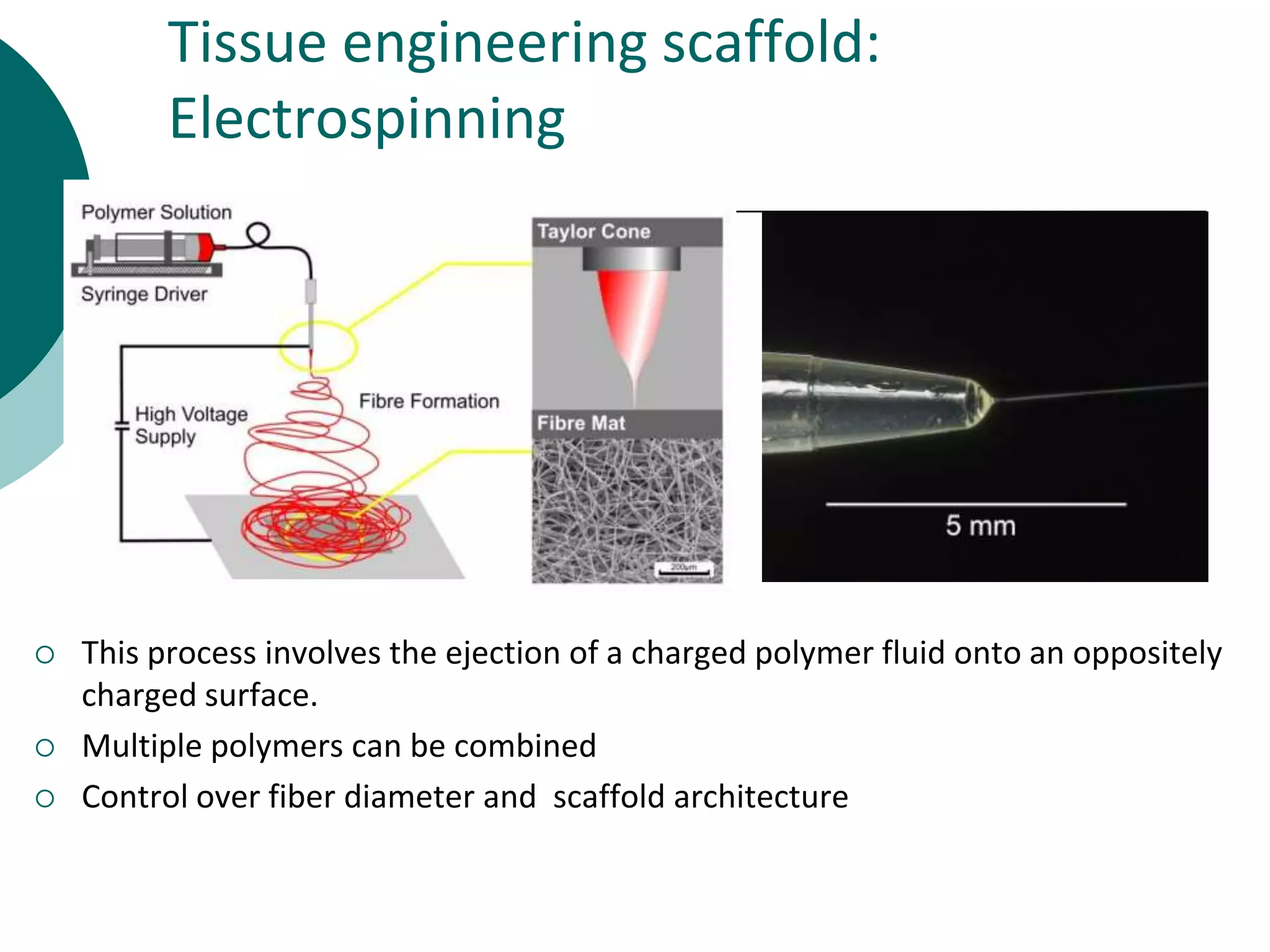
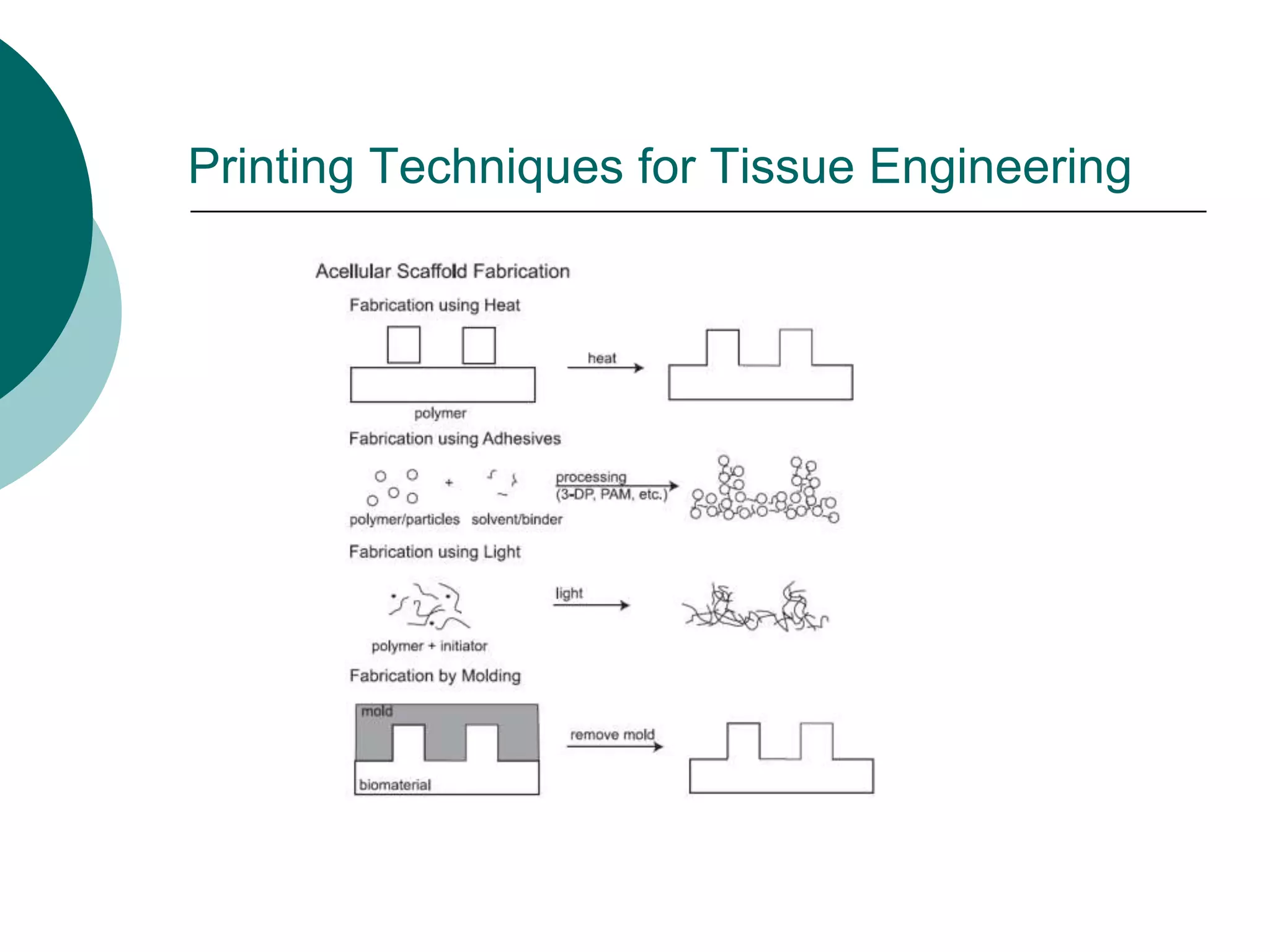
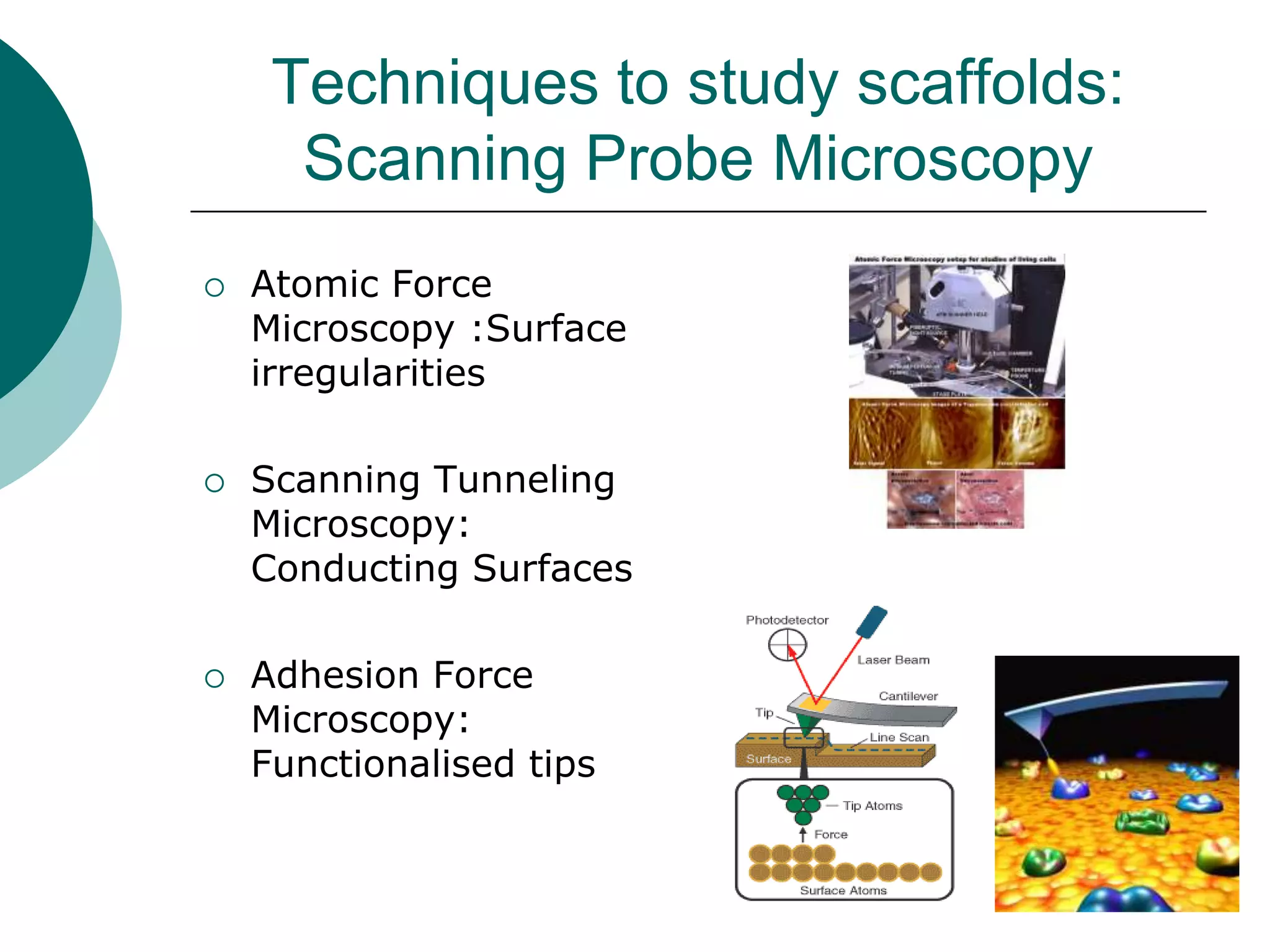
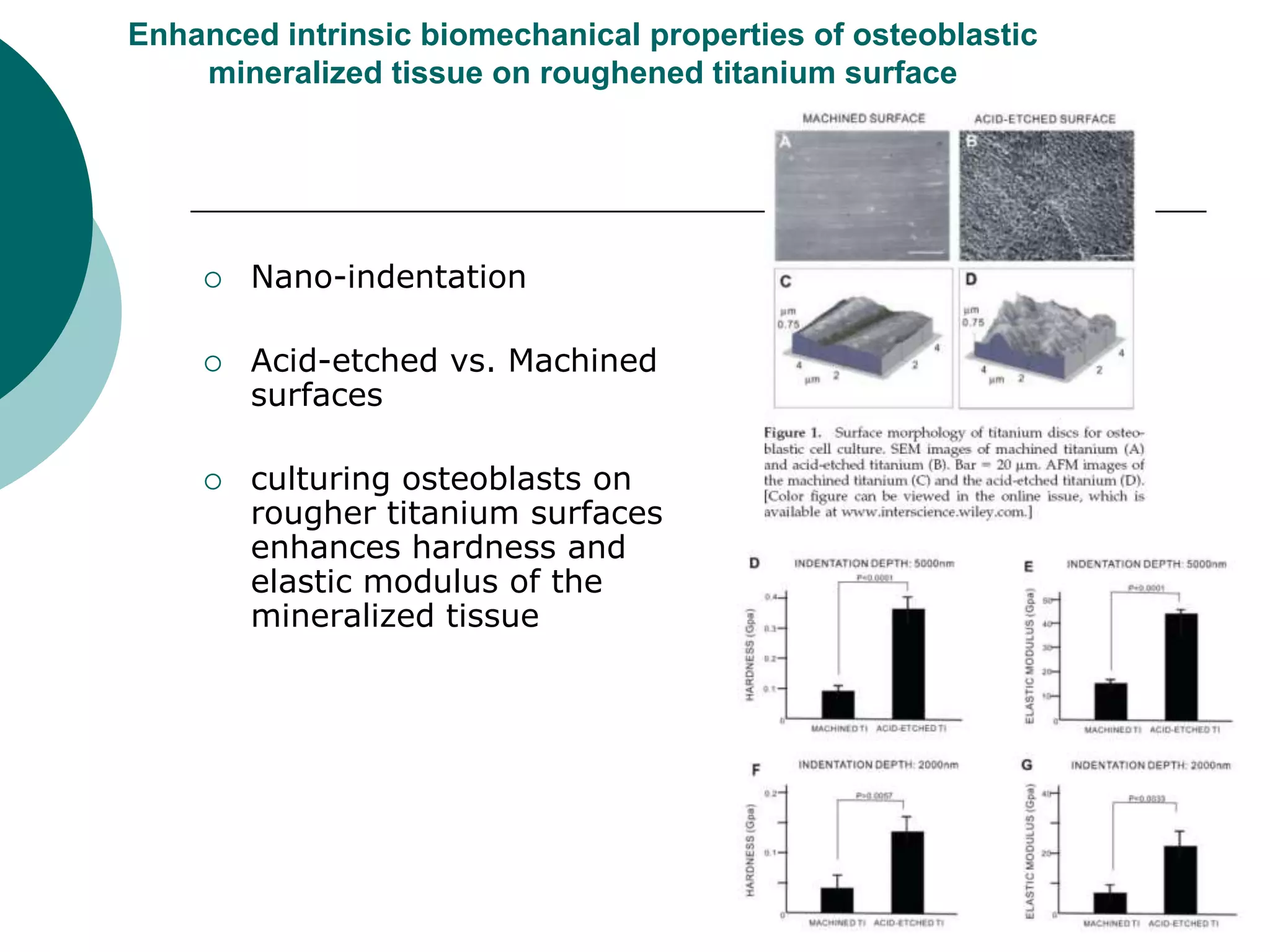
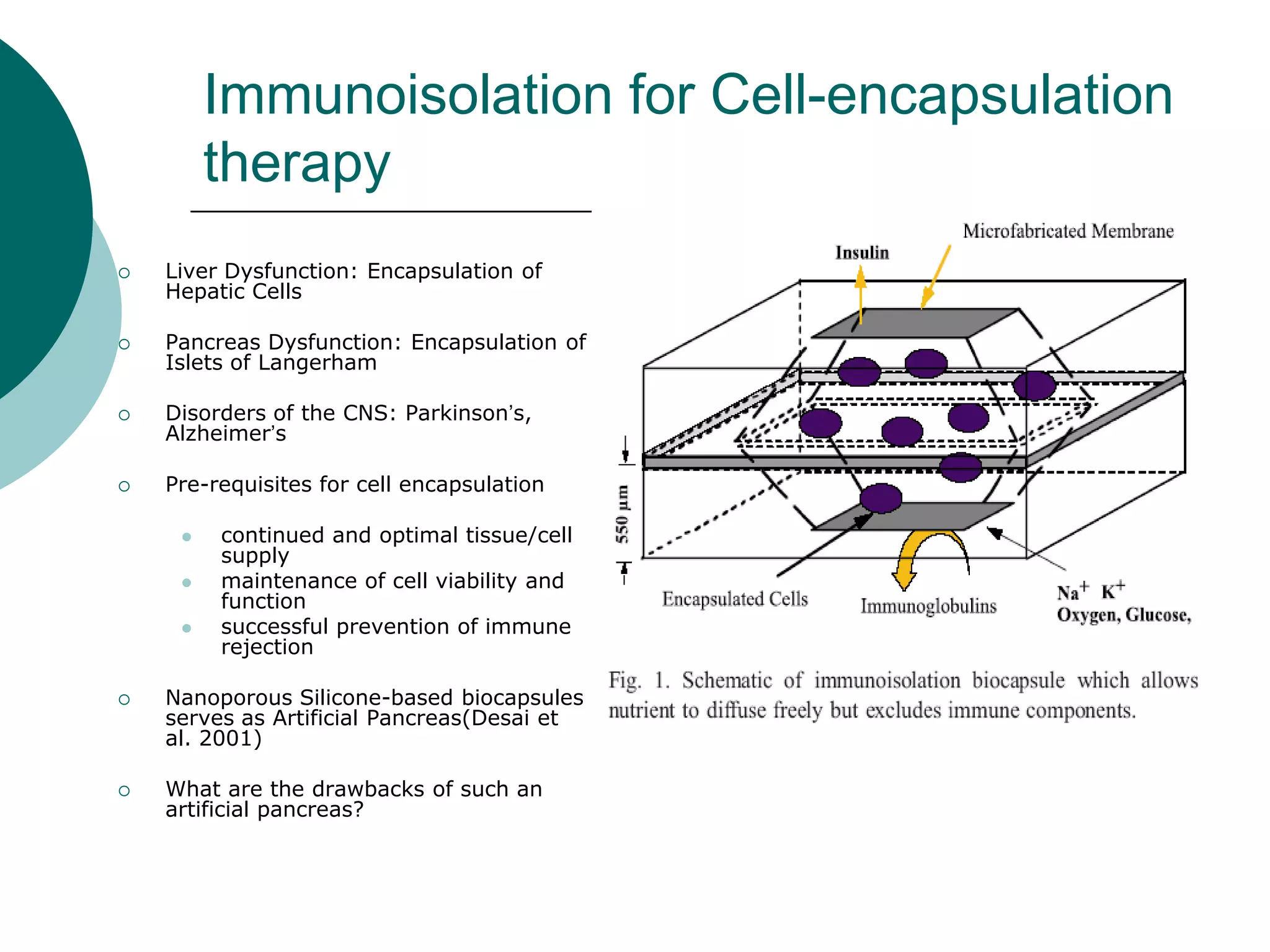
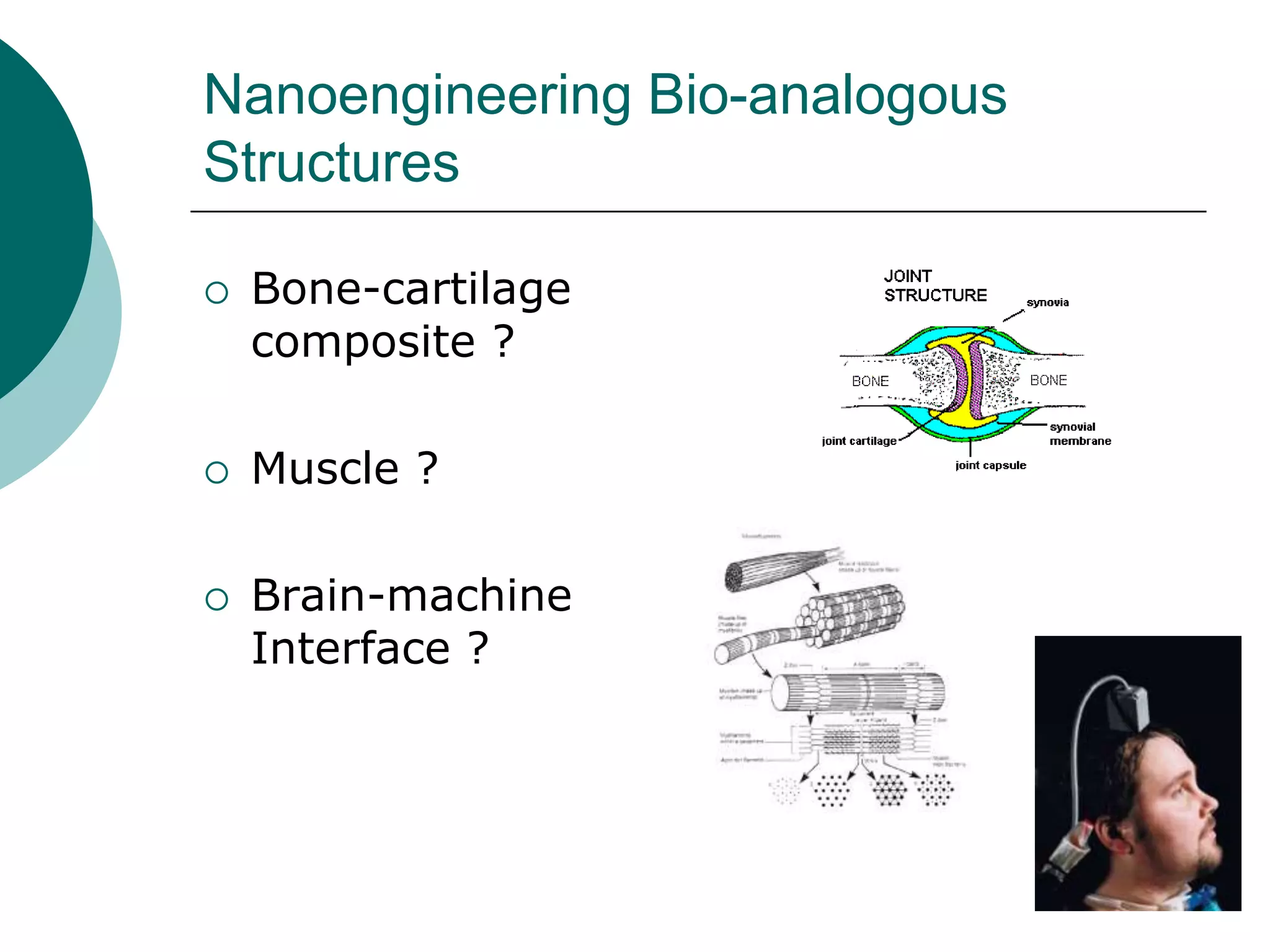
The document discusses research at the Liu Nanobionics Lab, which focuses on biomaterials, tissue engineering, and nanotechnology. The lab aims to address tissue damage from disease or injury by developing regenerative approaches rather than just replacement. This includes designing scaffolds, surface modifications, and cell encapsulation techniques to facilitate tissue regeneration. The goal is to shift from static tissue replacement to stimulating the body's natural healing abilities.

![Biomaterials
Biomaterials encompasses aspects of
medicine, biology, chemistry,
engineering and materials science.
Biomaterials are : “Non-viable
materials used in a medical devices
intended to interact with biological
systems” [D.F. Williams, 1987]](https://image.slidesharecdn.com/13-biomaterials-140129145925-phpapp02/75/13-biomaterials-2-2048.jpg)