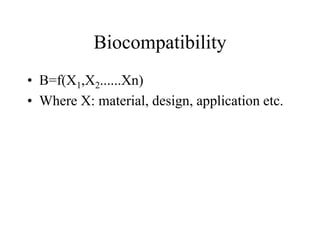
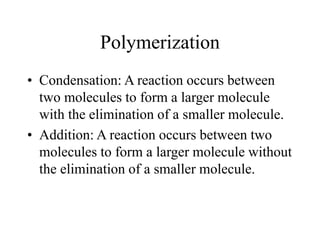
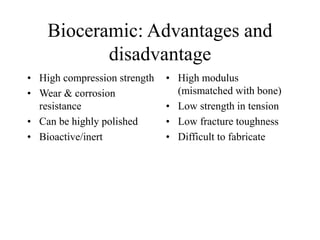
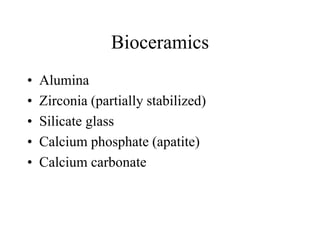
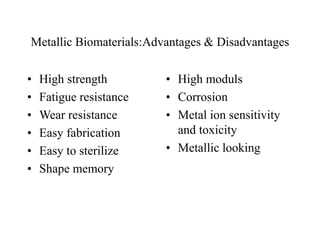
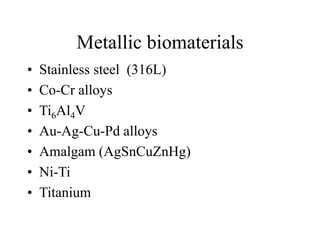
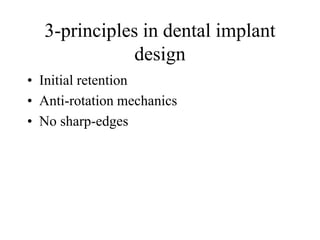
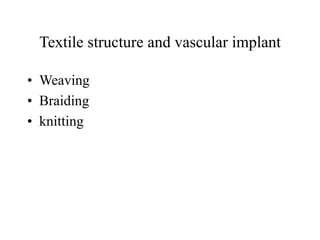
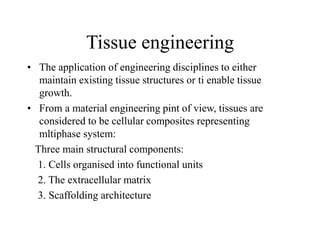
Calcium phosphate cement (CPC) is a synthetic bone graft material invented in 1986 consisting of tetracalcium phosphate and dicalcium phosphate anhydrous powders. When mixed with water, it forms a workable paste that hardens within 20 minutes to a nanocrystalline hydroxyapatite structure, which is biocompatible and osteoconductive. Over time, CPC is resorbed and replaced with new bone. It has advantages over pre-formed ceramics as the paste can be sculpted and its structure promotes bone growth. Recent work focuses on improving mechanical properties, making premixed versions, and seeding cells and growth factors into the cement.