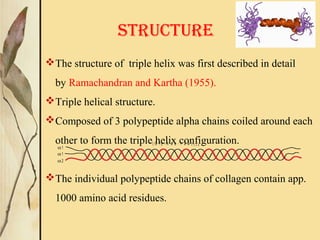
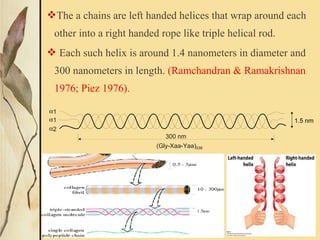
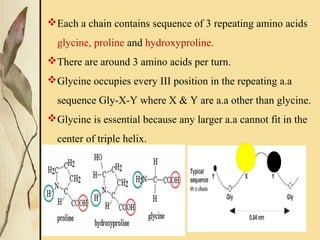
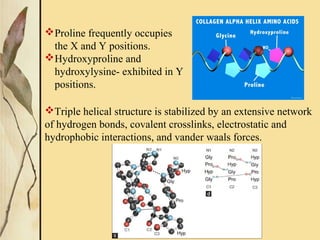
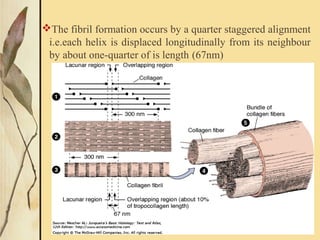
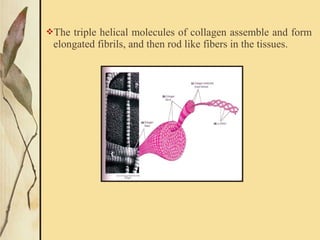
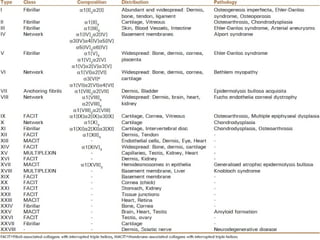
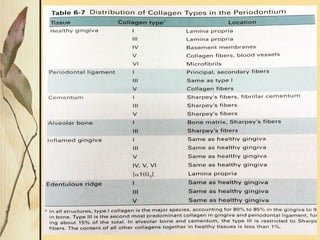
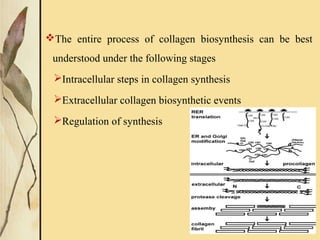
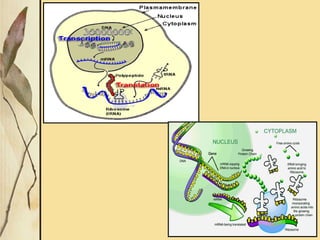
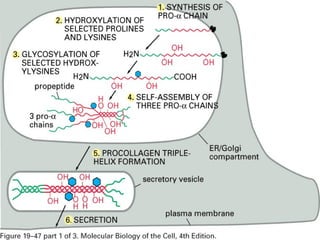
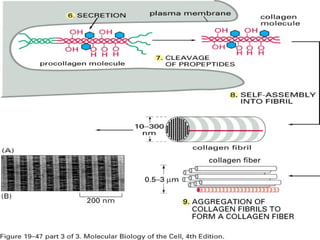
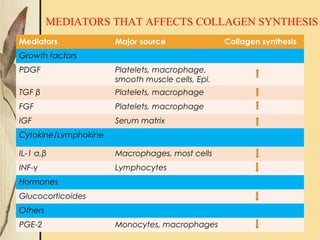
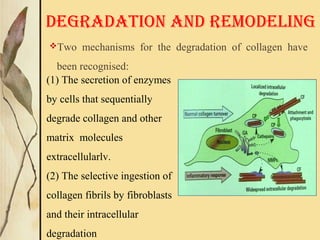
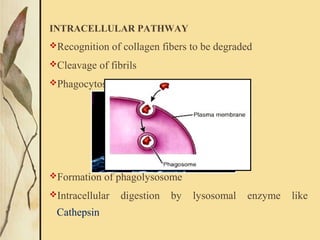
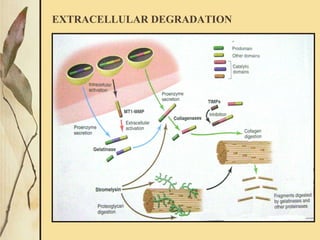
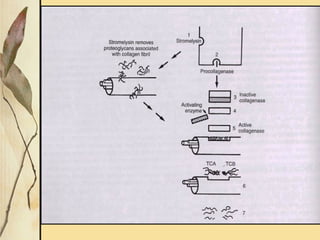
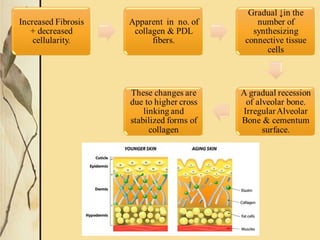
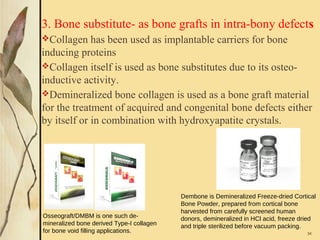
This document provides an overview of collagen, including its structure, types, synthesis, degradation, and uses in periodontics. Collagen is the most abundant protein in mammals, forms strong fibers, and is a major component of tissues like skin, bone, and teeth. It has a unique triple helical structure composed of three polypeptide chains that gives it great tensile strength. There are many types of collagen with varying distributions and roles. Collagen synthesis and degradation is tightly regulated, and pathological changes can occur in conditions like periodontitis. Collagen is used in periodontics for drug delivery, tissue augmentation, and as a bone substitute.