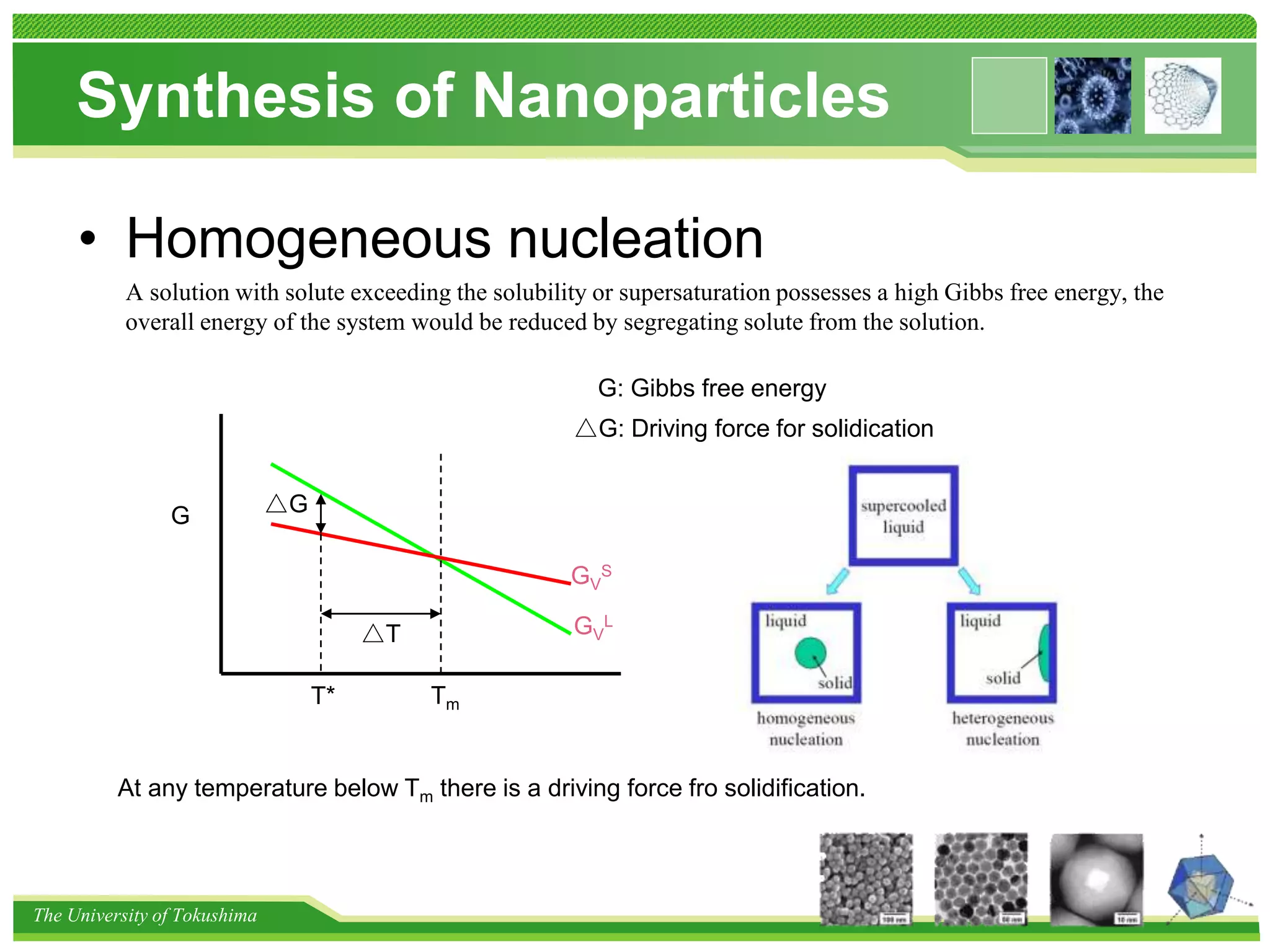
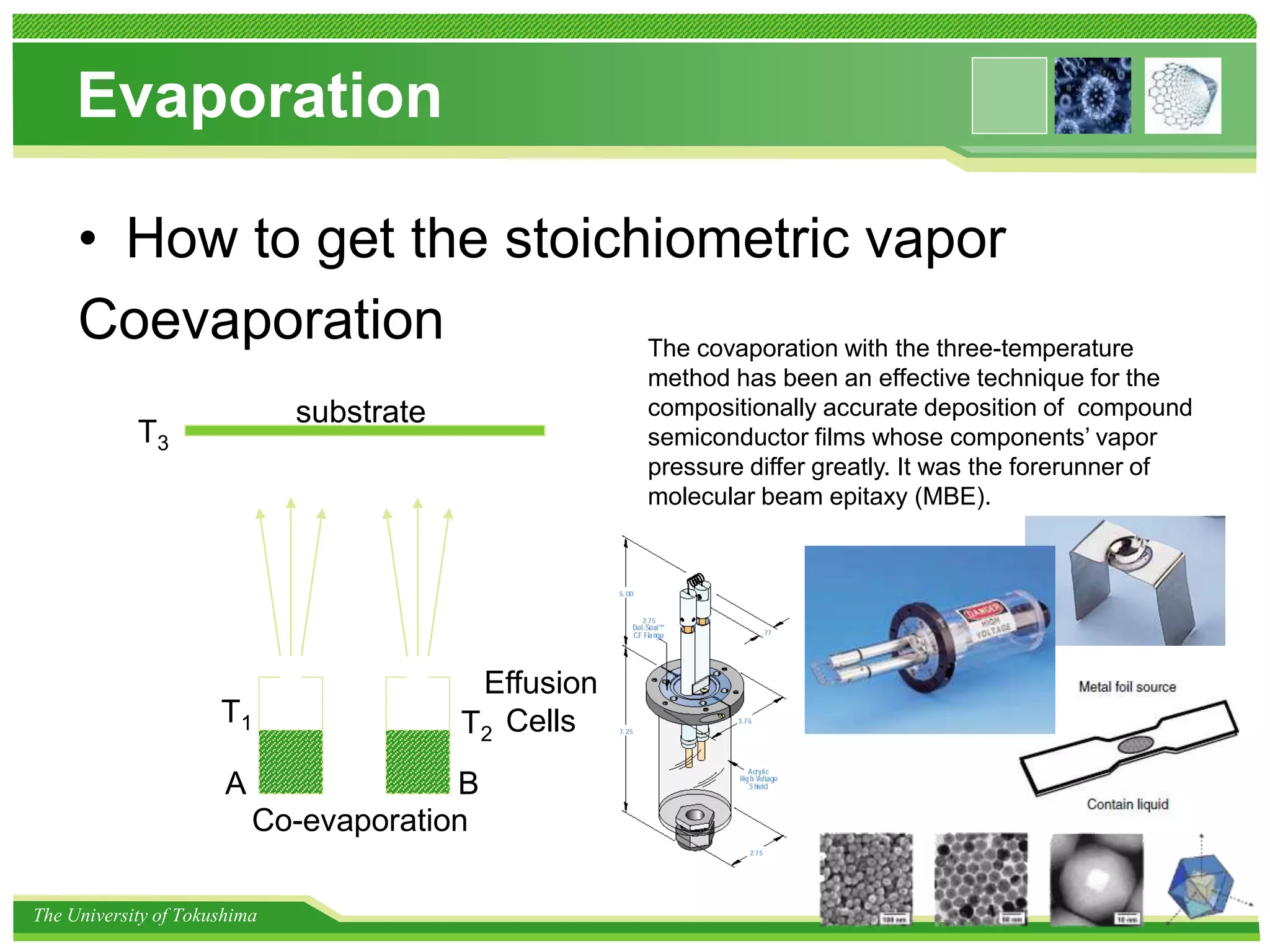
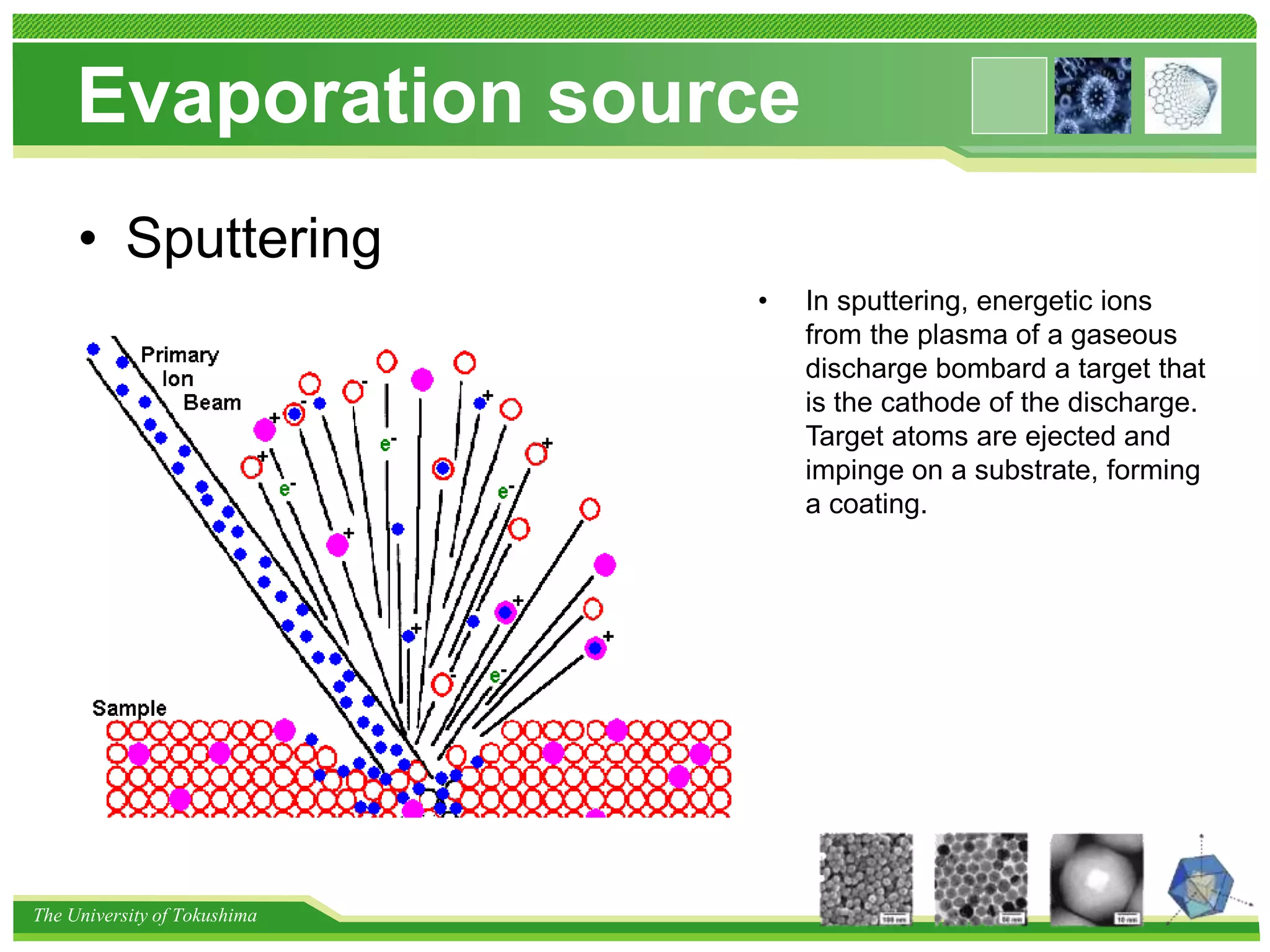
The document discusses nanomaterial synthesis methods. It begins with an introduction to nanotechnology and challenges in the field. It then covers bottom-up and top-down approaches to nanomaterial synthesis. Specific synthesis methods covered include evaporation and condensation growth, lithography technology, and methods for creating nano-composites. A variety of nanoparticle synthesis techniques are also discussed.