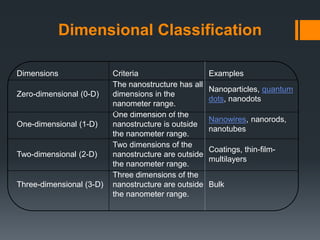
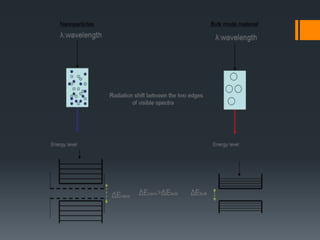
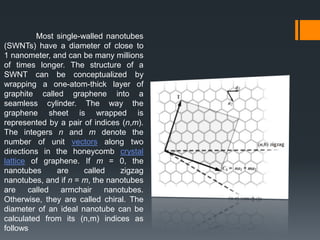
Nanotechnology harnesses unusual behaviors of materials at the nanoscale (1-100 nanometers) to achieve scientific and practical results. Materials behave differently at the nanoscale, enabling applications in many fields. Nanotechnology is used in electronics, sunscreens, and other products, and is predicted to significantly grow the number of related jobs. Nanostructures are classified by their dimensions, with zero-dimensional structures having all dimensions in the nanoscale and higher dimensions having some dimensions outside this range. Properties like surface area to volume ratio and quantum confinement explain how size at the nanoscale impacts behavior.