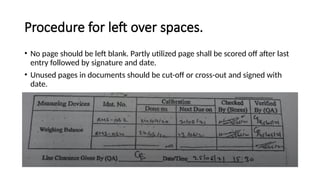
The document outlines a Corporate Standard Operating Procedure (CSOP) for Good Documentation Practice (GDP) in the pharmaceutical industry, specifying procedures for accurate data recording across various essential documents. It details responsibilities of personnel involved in cGMP activities and emphasizes the importance of real-time data entries, traceability, and proper correction methods for documentation. The document also states best practices for maintaining integrity and reliability of recorded data and prohibits certain incorrect documentation methods.