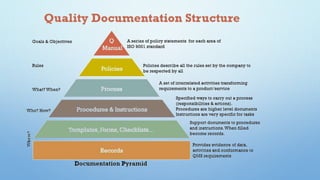
The document provides guidelines for quality assurance documentation in the pharmaceutical industry. It outlines that documentation is essential to define specifications and procedures, ensure personnel know their responsibilities, and provide an audit trail. The summary is as follows:
1) Documentation should exist for all quality assurance aspects and be designed, prepared, reviewed and distributed carefully.
2) Documents must be unambiguous and include titles, contents, and be easy to check. Reproduced documents must be clear and legible.
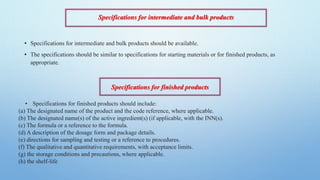
3) Documentation such as specifications, batch records, testing procedures and analysis records should include details like product names, batch numbers, test methods and results to ensure traceability.