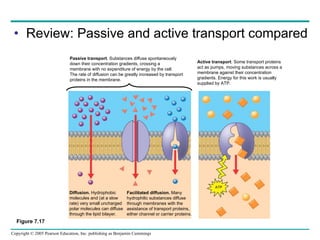
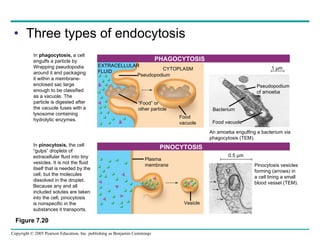
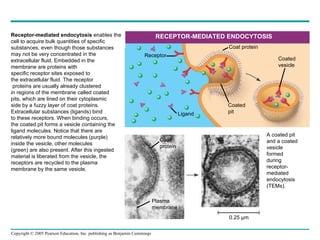
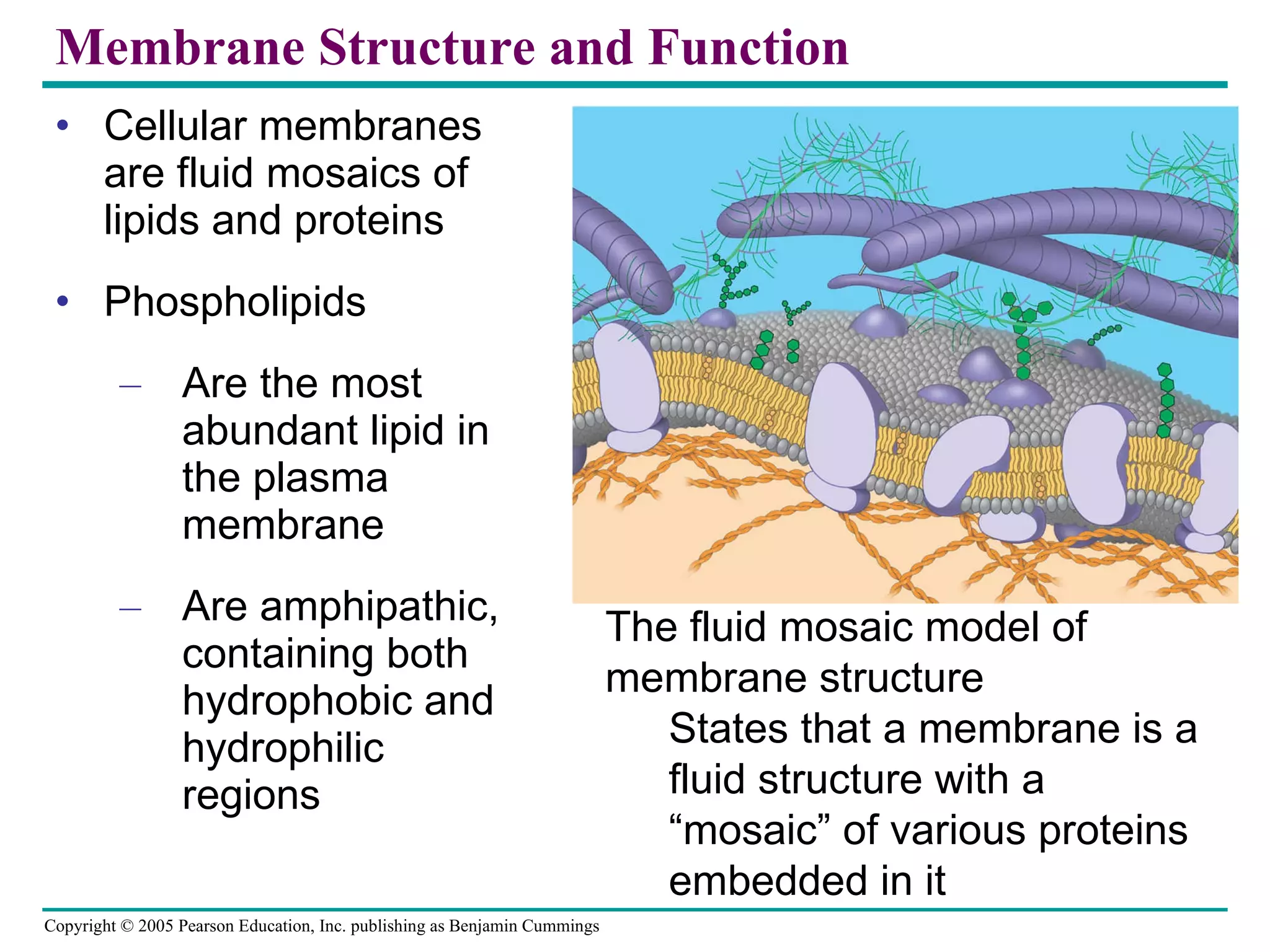
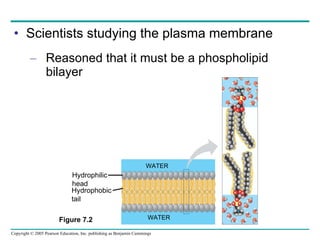
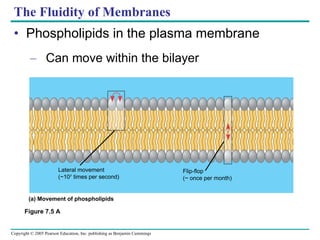
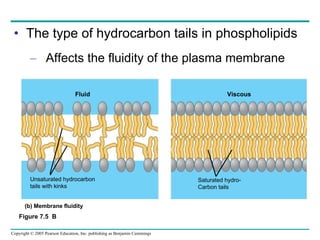
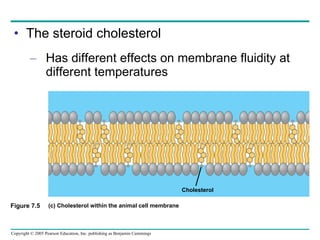
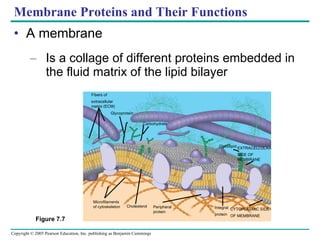
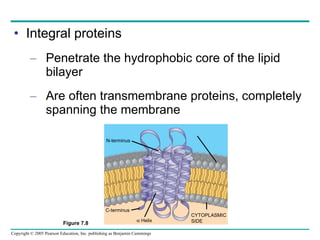
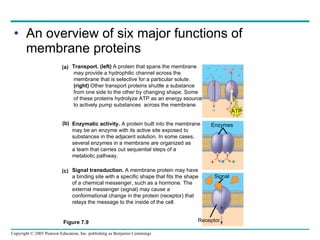
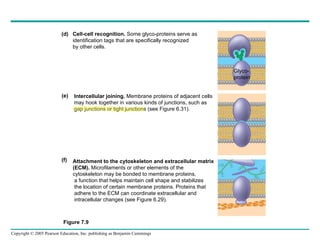
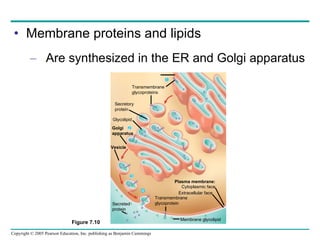
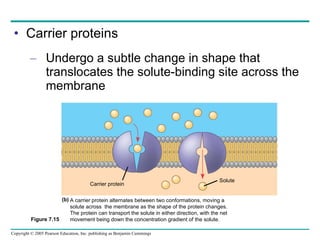
Cellular membranes are fluid mosaics of lipids and proteins. Phospholipids are the main lipid and form a bilayer that is fluid and allows movement of proteins within it. Membrane proteins have various functions including transport, cell-cell recognition, and attachment to other cell structures. The membrane is selectively permeable due to its structure and specialized transport proteins that allow passage of substances through the membrane.


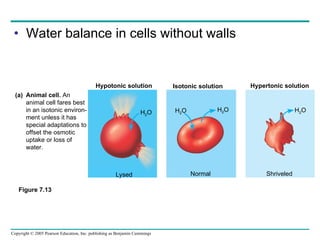
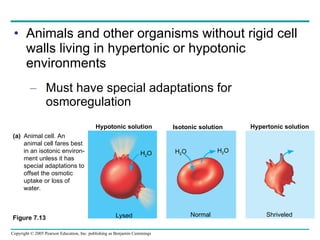
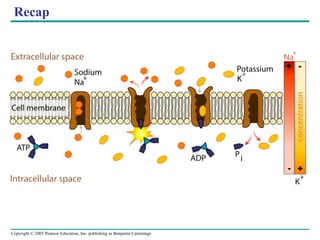
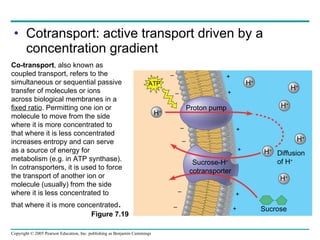
![The sodium-potassium pump Is one type of active transport system Figure 7.16 P P i EXTRACELLULAR FLUID 4 5 6 3 Na+ binding stimulates phosphorylation by ATP. 2 Na + Cytoplasmic Na + binds to the sodium-potassium pump. 1 K + is released and Na + sites are receptive again; the cycle repeats. 3 Phosphorylation causes the protein to change its conformation, expelling Na + to the outside. Extracellular K + binds to the protein, triggering release of the Phosphate group. Loss of the phosphate restores the protein’s original conformation. CYTOPLASM [Na + ] low [K + ] high Na + Na + Na + Na + Na + P ATP Na + Na + Na + P ADP K + K + K + K + K + K + [Na + ] high [K + ] low](https://image.slidesharecdn.com/07-membranestext-100912180025-phpapp01/85/07-membranes-text-35-320.jpg)