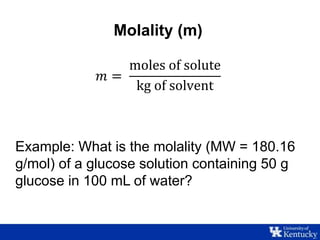
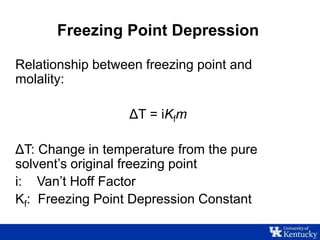
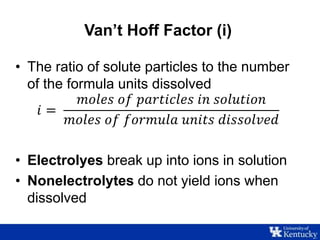
This document discusses freezing point depression and colligative properties. It defines key terms like molality, freezing point depression constant, and Van't Hoff factor. The goals are to determine the freezing point depression constant of water, examine the relationship between concentration and freezing point, and explore the effect of electrolytes. Students will prepare sugar solutions of varying concentration and one salt solution to compare the freezing point depression.