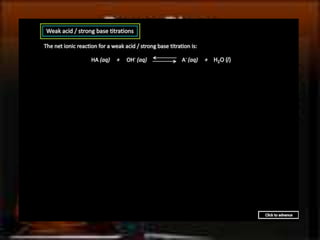
This document discusses acid-base titration, including definitions of acids and bases, strong vs weak acids and bases, and the technique of titration. It explains that a titration reaches the equivalence point when the moles of acid equals the moles of base, producing a neutral solution. This can be shown using the equation MAVA = MBVB, where M is molarity, V is volume, and A and B indicate acid and base. An example titration calculation is provided.






![Acid-Base Indicators
Finding the equivalence point of a titration
Use a pH meter
- Plot pH versus titrant volume
- Center vertical region = equivalence point
Use an Acid-Base Indicator
- Acid-Base Indicator = molecule that changes color based on pH
- Choose an indicator that changes color at the equivalence point
- End Point = when the indicator changes color. If you have chosen the
wrong indicator, the end point will be different than the eq. pt.
- Indicators are often Weak Acids that lose a proton (causing the color
change) when [OH-] reaches a certain concentration
HIn + OH- In- + H2O](https://image.slidesharecdn.com/acidbasetitration-141124015743-conversion-gate02/85/Acid-base-titration-7-320.jpg)