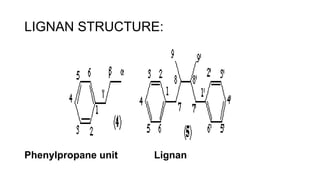
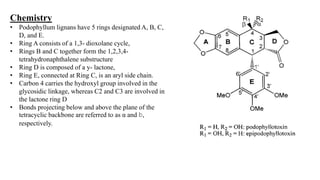
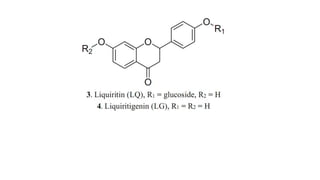
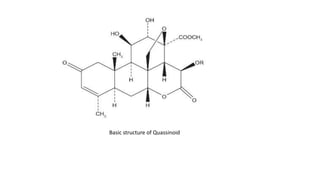
Lignans are phenylpropanoid dimers found widely in plants. Podophyllum lignans from Podophyllum species have anticancer and antiviral properties. Their structure contains five rings. Phenylpropane units in lignans are derived from the phenylpropanoid pathway. Secoisolariciresinol diglucoside is a prominent lignan in flaxseed that has antioxidant and antiplatelet effects. Quassinoids from Quassia species have insecticidal, antibacterial, and antitumor properties. Their basic structure contains a beta-carbolinium ion.