The document discusses acid-base equilibrium theories including Arrhenius, Brönsted-Lowry, and Lewis definitions, focusing primarily on their behavior in aqueous solutions. It outlines the distinction between strong and weak acids and bases, along with relevant equations for calculating dissociation constants (Ka and Kb) and pH. Additionally, it covers concepts like hydrolysis of salts and titration of acids and bases, including examples and calculations for determining specific acid strengths.








![Kw and pHWater is weakly dissociated and the equilibrium can be shown like thisH2O + H2O = H3O+ + OHH2O = H+ + OH-Kw = [H+][OH-]Kw = 10-14 at 250C for water H2O = H++ OH--x +x +xKw = x2x = = 10-7](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-9-320.jpg)
![pHpH = -log[H+]pOH = -log[OH-]For water pH = -log[10-7] = - [-7log 1o] = 7Similarly pOH = 7pH + pOH = 14pOH = 14-pHpH = 14 - pOH](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-10-320.jpg)


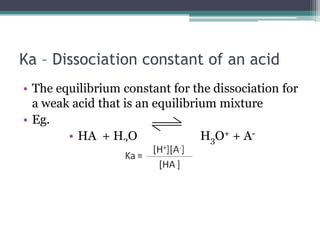

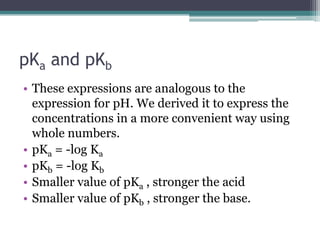
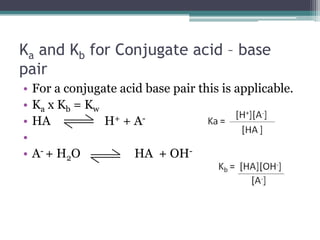


![Calculations involving KaCalculate the value of Ka for a specific acid from the [H+] or pH of a solution for which we also know the initial concentration of the acidCalculate the equilibrium concentrations of H+ and A- from the initial concentration of a specific weak acid and its Ka value](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-19-320.jpg)
![Calculations involving KbCalculate the value of Ka for a specific acid from the [OH-] or pOH of a solution for which we also know the initial concentration of the base or from pH as pOH = 14 - pHCalculate the equilibrium concentrations of [H+ ]or [OH-]from the initial concentration of a specific weak base and its Kb value (Or what is almost the same is calculating the pH or pOH from the values of Kb and initial concentration of base)](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-20-320.jpg)







![#1Determine the [H+] ion concentration from pHMake Ice table sand substitute the concentration of H+ in the table and complete it to get the equilibrium concentration of HA, H+ and A-Use the values to determine the Ka for the acid.](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-28-320.jpg)
![Calculating [H+], pH from Ka for weak acid #2The concentration of vinegar HC2H3O2 was found to be 0.75 M acetic acid, Calculate the value for the [H+] ion concentration and pH for this acid solution if Ka for acetic acid is 1.8 x 10-5](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-29-320.jpg)
