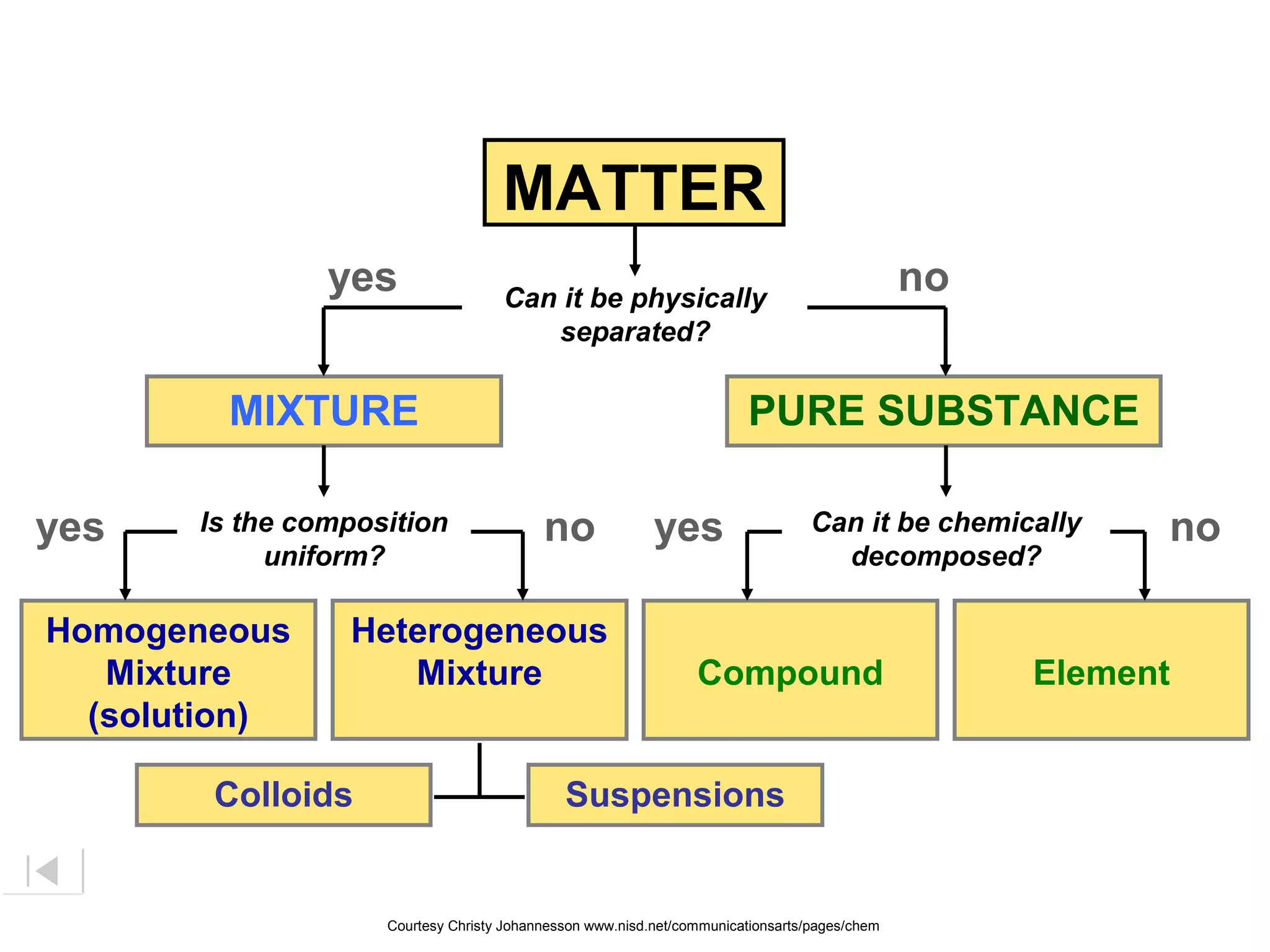
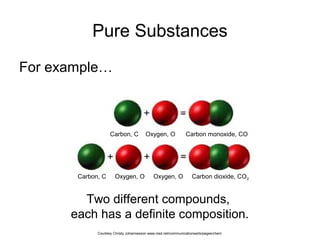
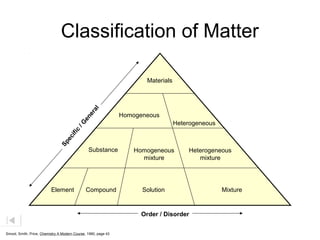
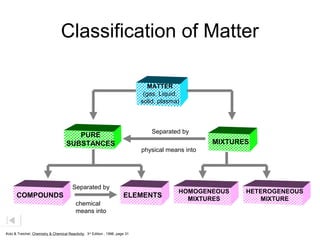
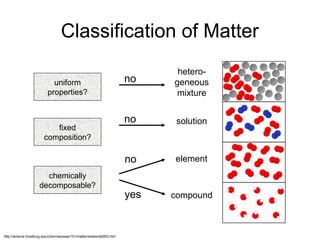
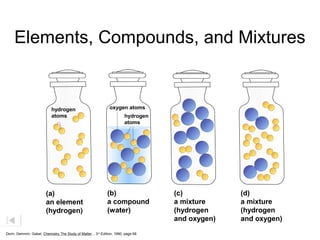
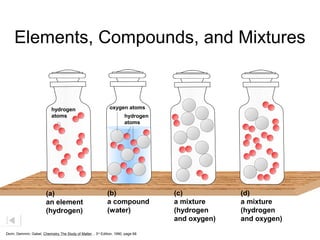
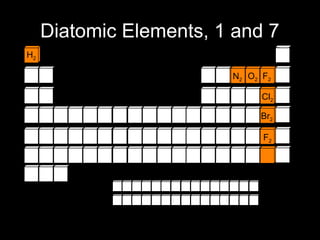
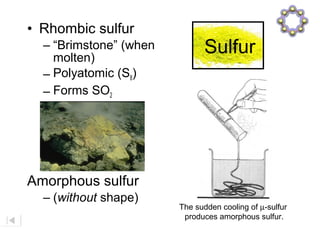
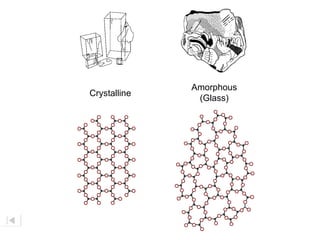
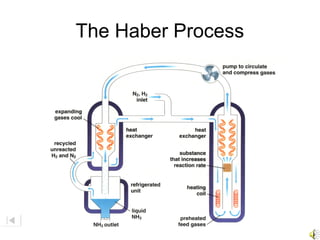
Here are brief explanations of the key concepts:
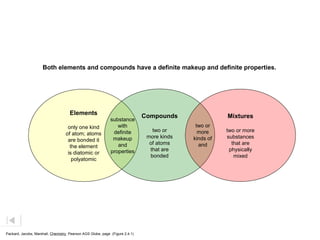
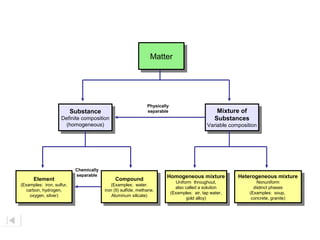
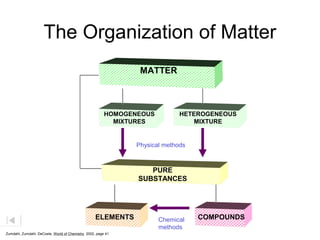
- The composition of an element is fixed because elements are pure substances made of only one type of atom.
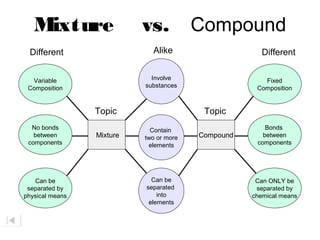
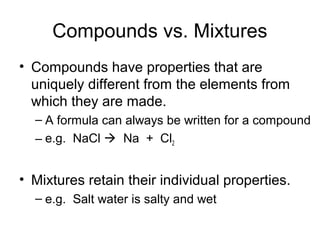
- The composition of a compound is also fixed, but compounds contain two or more elements combined in a fixed ratio.
- Properties of mixtures can vary because mixtures are combinations of two or more substances that are not chemically combined. Their compositions are not fixed.
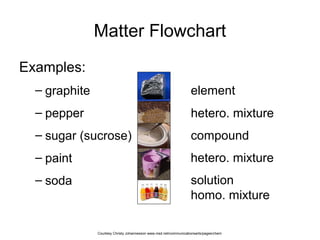
- Mixtures can be classified as solutions, suspensions, or colloids based on whether the mixed substances are uniformly dispersed (solutions), settle over time (suspensions), or are dispersed with particles too small to settle but large enough to scatter light (colloids).
- Every sample of