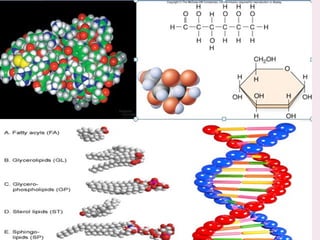
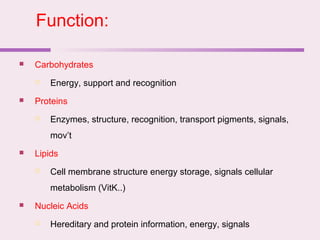
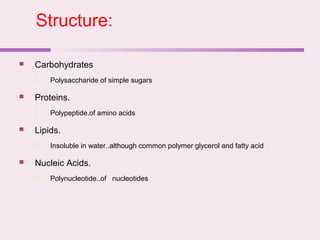
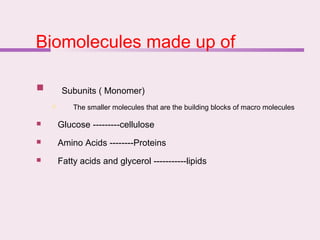
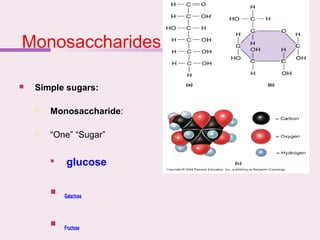
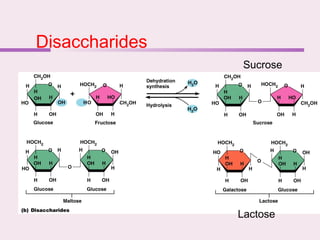
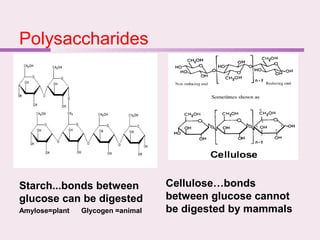
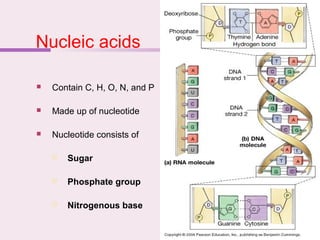
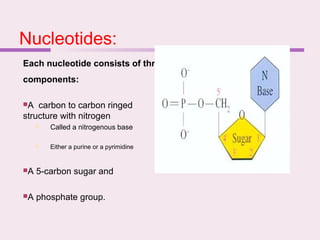
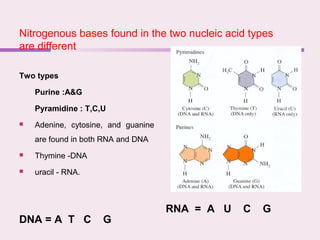
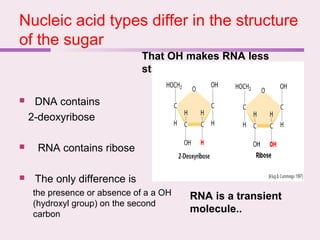
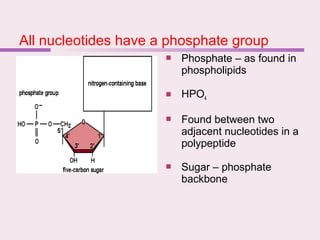
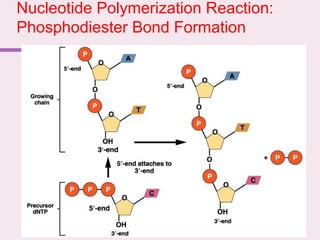
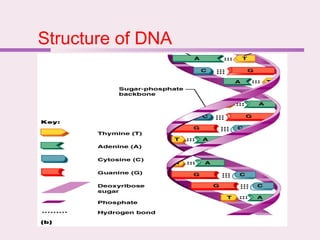
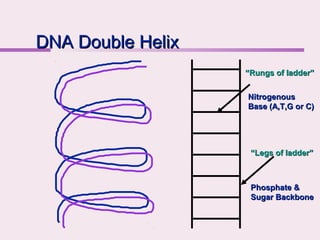
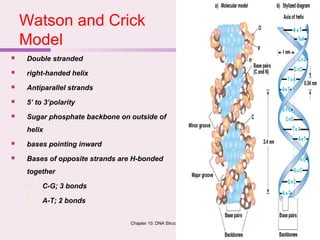
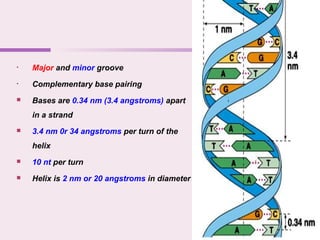
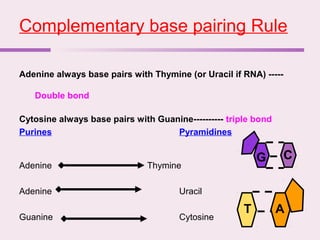
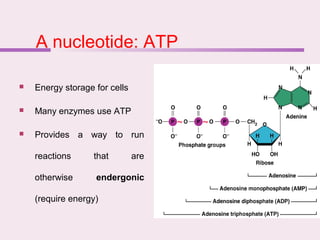
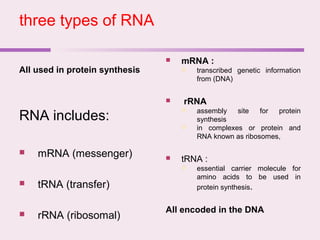
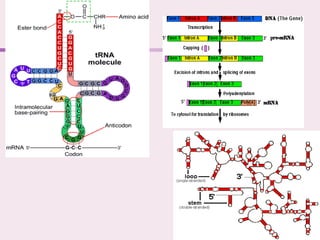
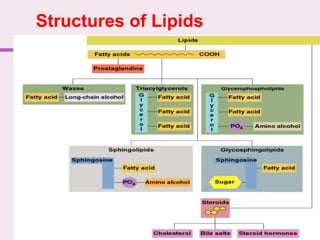
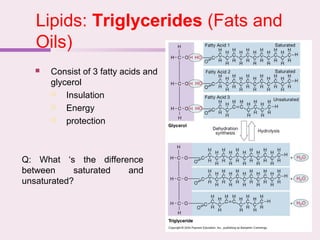
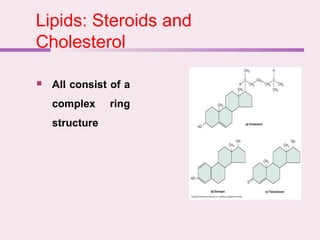
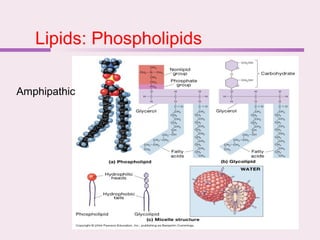
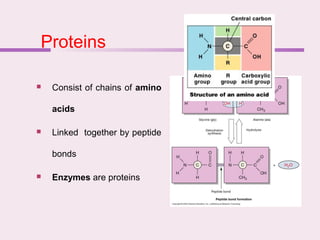
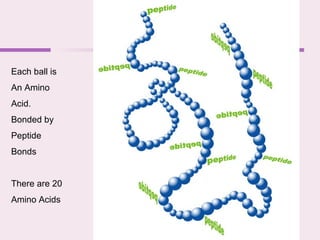
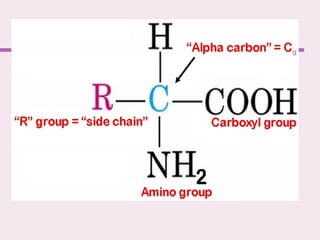
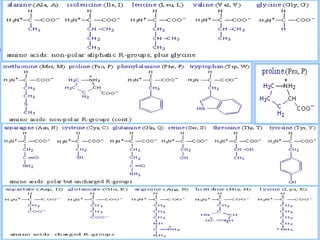
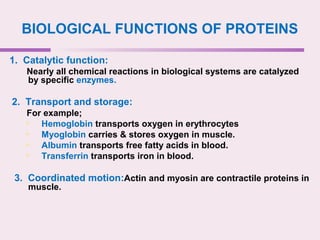
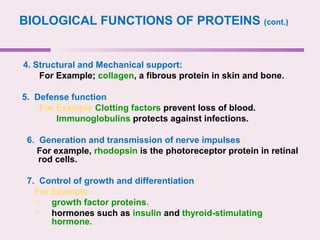
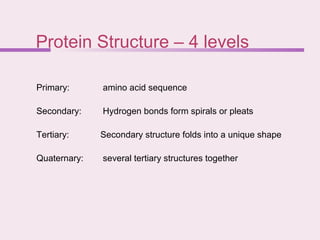
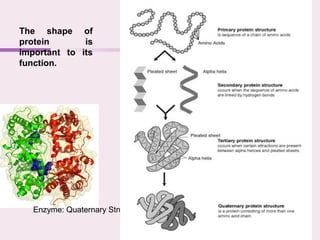
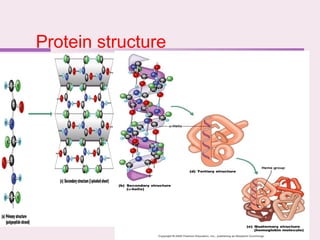
The document discusses the key biomolecules - carbohydrates, lipids, proteins, and nucleic acids - that make up living organisms. It provides examples of each type of biomolecule and explains their structure and functions. Carbohydrates include sugars and starches, lipids include fats and phospholipids, proteins are made of amino acids in complex structures related to their function, and nucleic acids like DNA and RNA carry genetic information and aid in protein synthesis. These biomolecules are the basic building blocks and materials that make up living cells and perform essential functions.