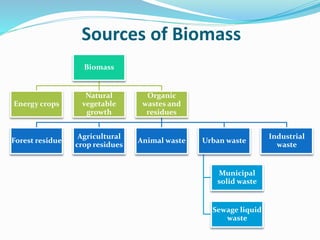
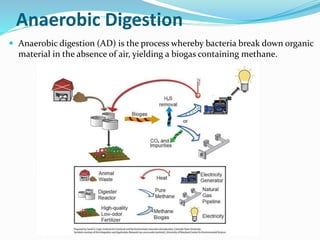
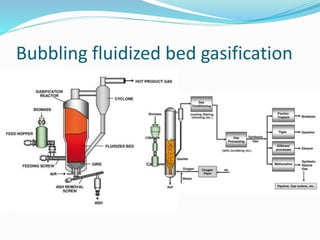
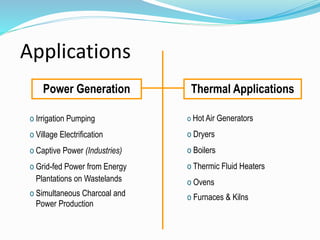
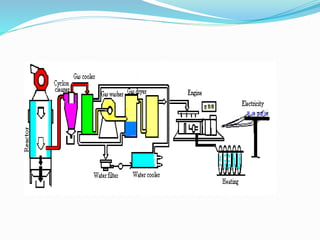
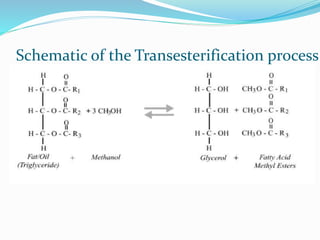
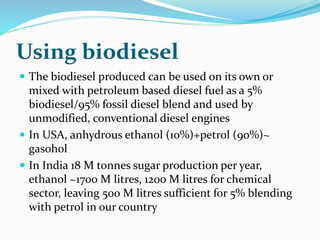
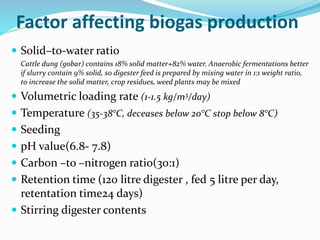
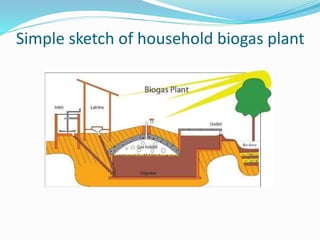
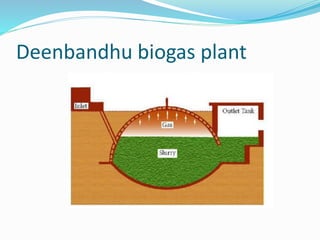
This document discusses biomass and its uses as an energy source. It defines biomass as biological material from living or recently living organisms composed primarily of carbon, hydrogen, oxygen, nitrogen and other elements. Biomass is obtained from various sources including plants, animals, and waste materials. The document discusses different types of biomass such as virgin wood, energy crops, agricultural residues, food waste, and industrial waste. It also discusses various thermal and chemical conversion processes that can be used to convert biomass into energy sources like heat, electricity, biofuels and biogas. These conversion processes include combustion, gasification, pyrolysis, anaerobic digestion, fermentation and trans esterification.