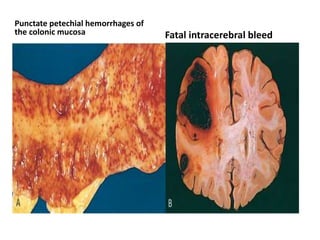
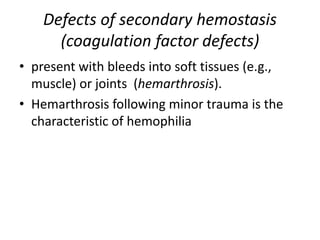
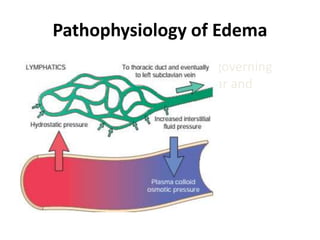
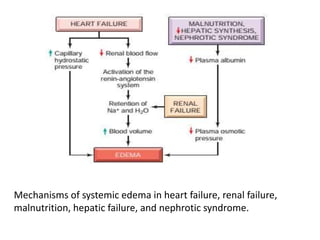
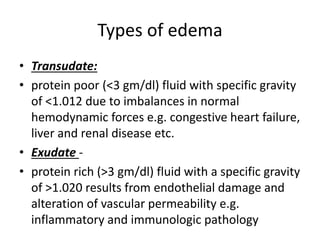
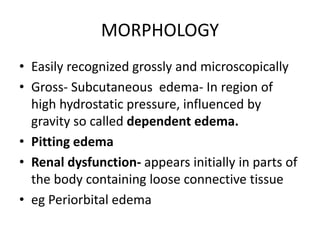
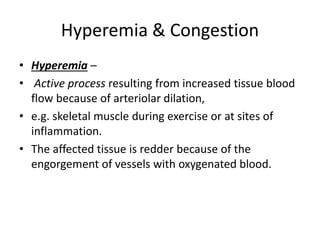
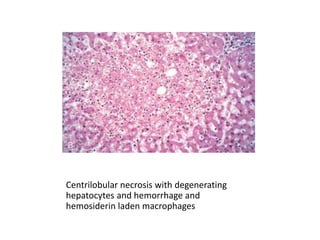
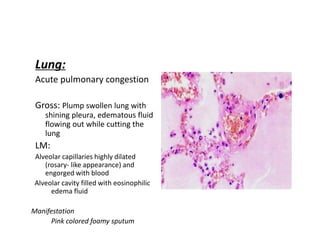
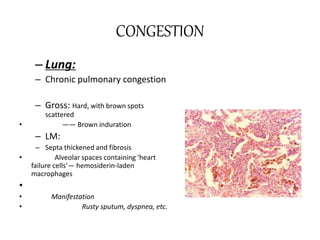
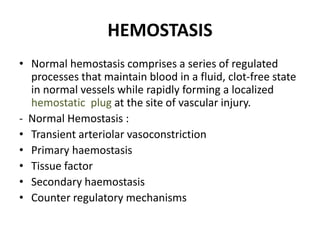
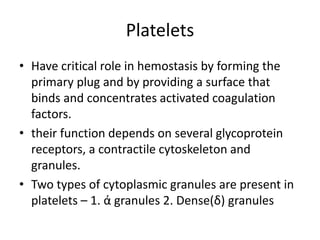
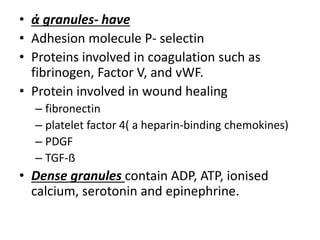
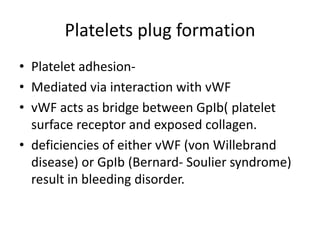
This document discusses hemodynamic disorders and edema. It begins by defining the normal composition of body water and the three body compartments it is contained in. It then defines edema as excess fluid in the interstitial tissue space and describes different types of edema based on location. The pathophysiology of edema involves either increased hydrostatic pressure or reduced plasma osmotic pressure. Specific causes of edema are discussed like congestive heart failure, liver disease, and malnutrition. The document also covers morphology, hemorrhage, congestion, hemostasis, and hemorrhagic disorders.






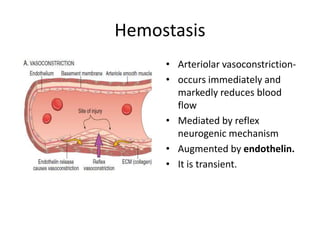
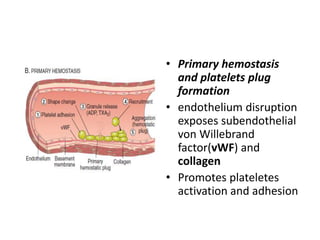
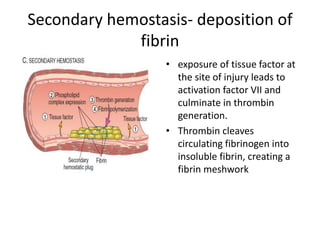
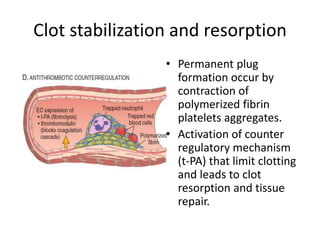

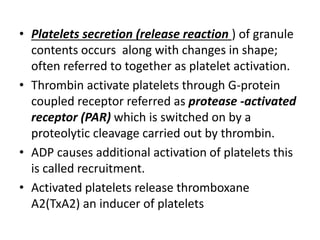
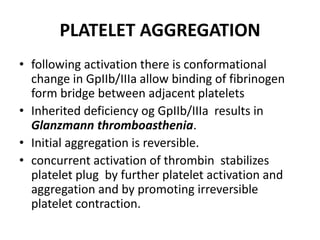
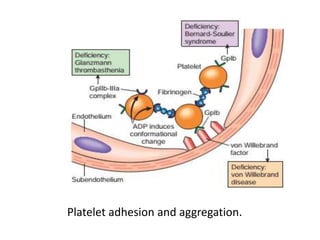

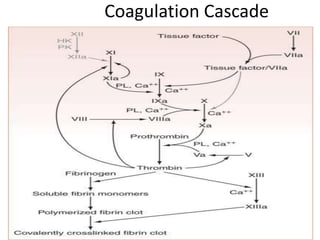
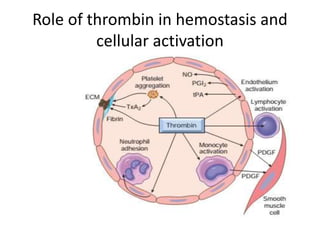
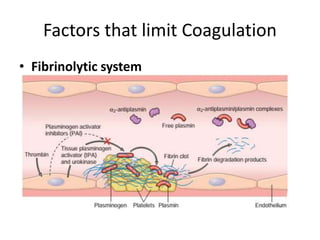
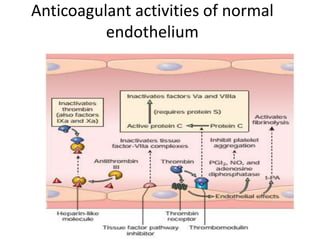
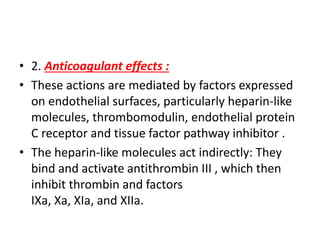
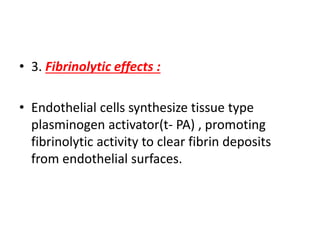
![Antithrombotic properties :
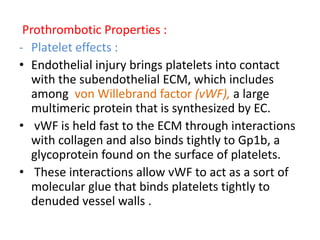
• Antiplatelet effects :
• Intact endothelium prevents platelets (and
plasma coagulation factors) from engaging the
highly thrombogenic subendothelial ECM.
• Nonactivated platelets do not adhere to
normal endothelium; even with activated
platelets, prostacyclin (i.e., prostaglandin I2
[PGI2]) and nitric oxide produced by
endothelium impede their adhesion.](https://image.slidesharecdn.com/hemodynamicdisorder-1-150501132509-conversion-gate02/85/Hemodynamic-disorder-1-40-320.jpg)