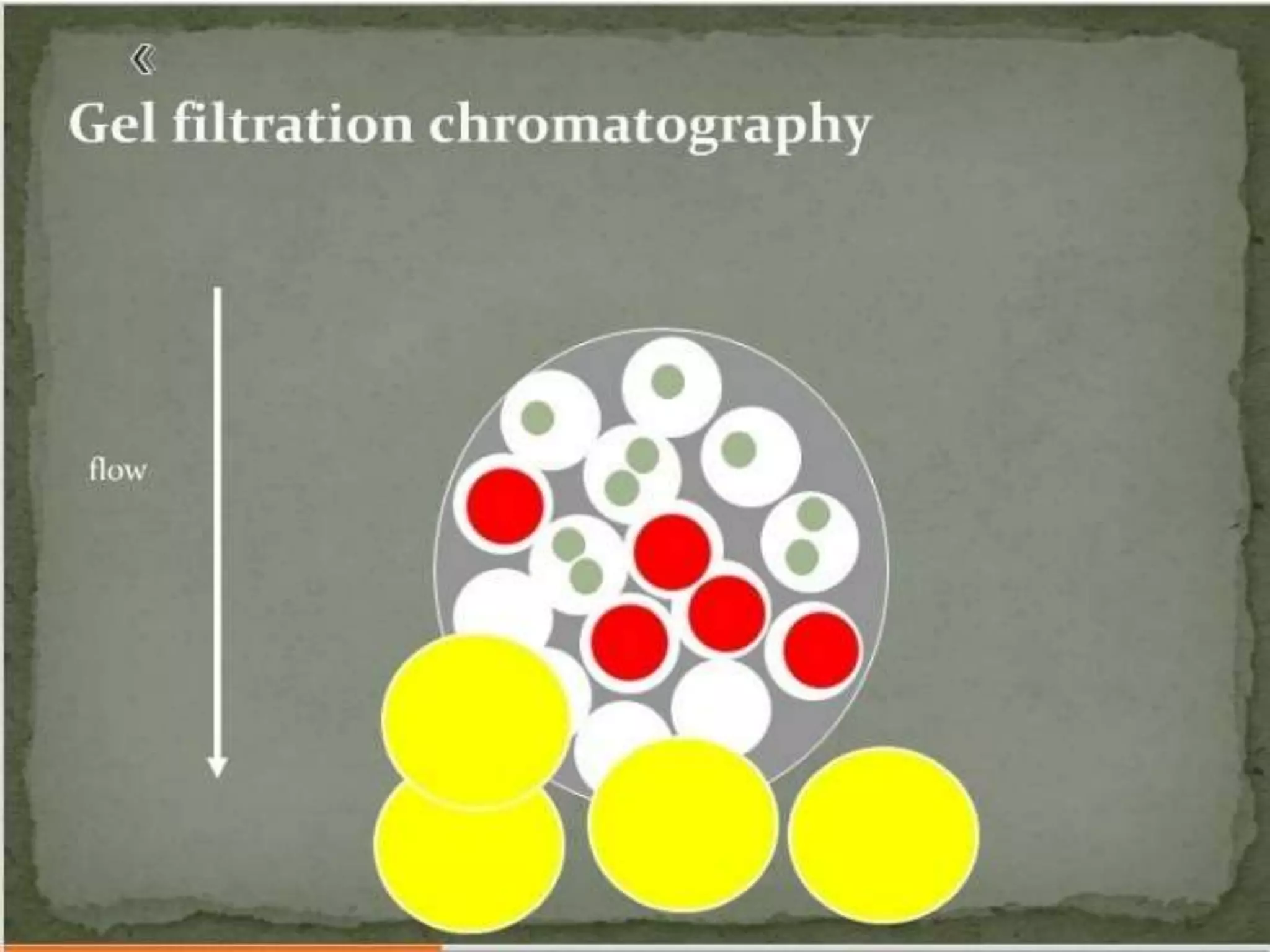
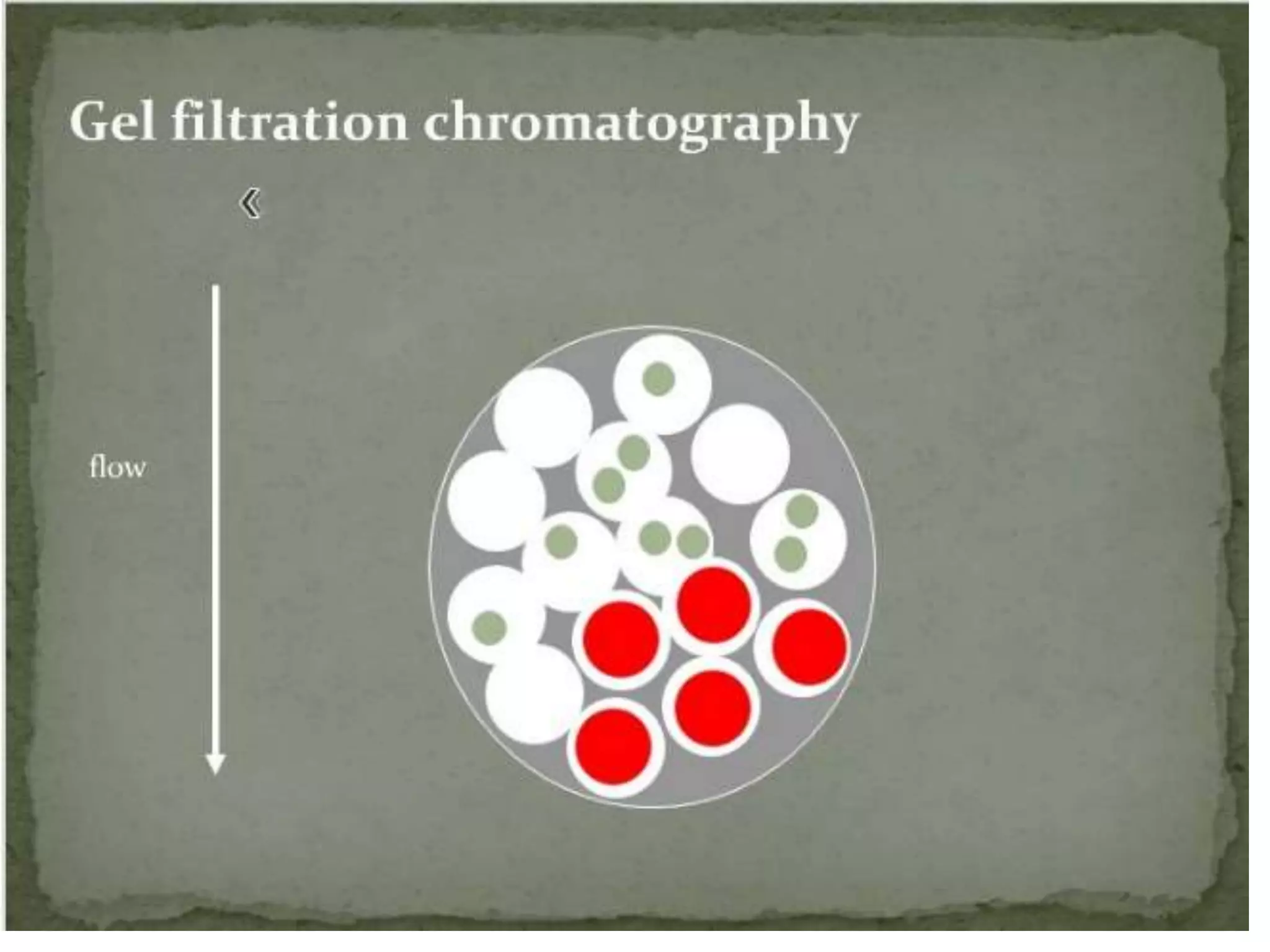
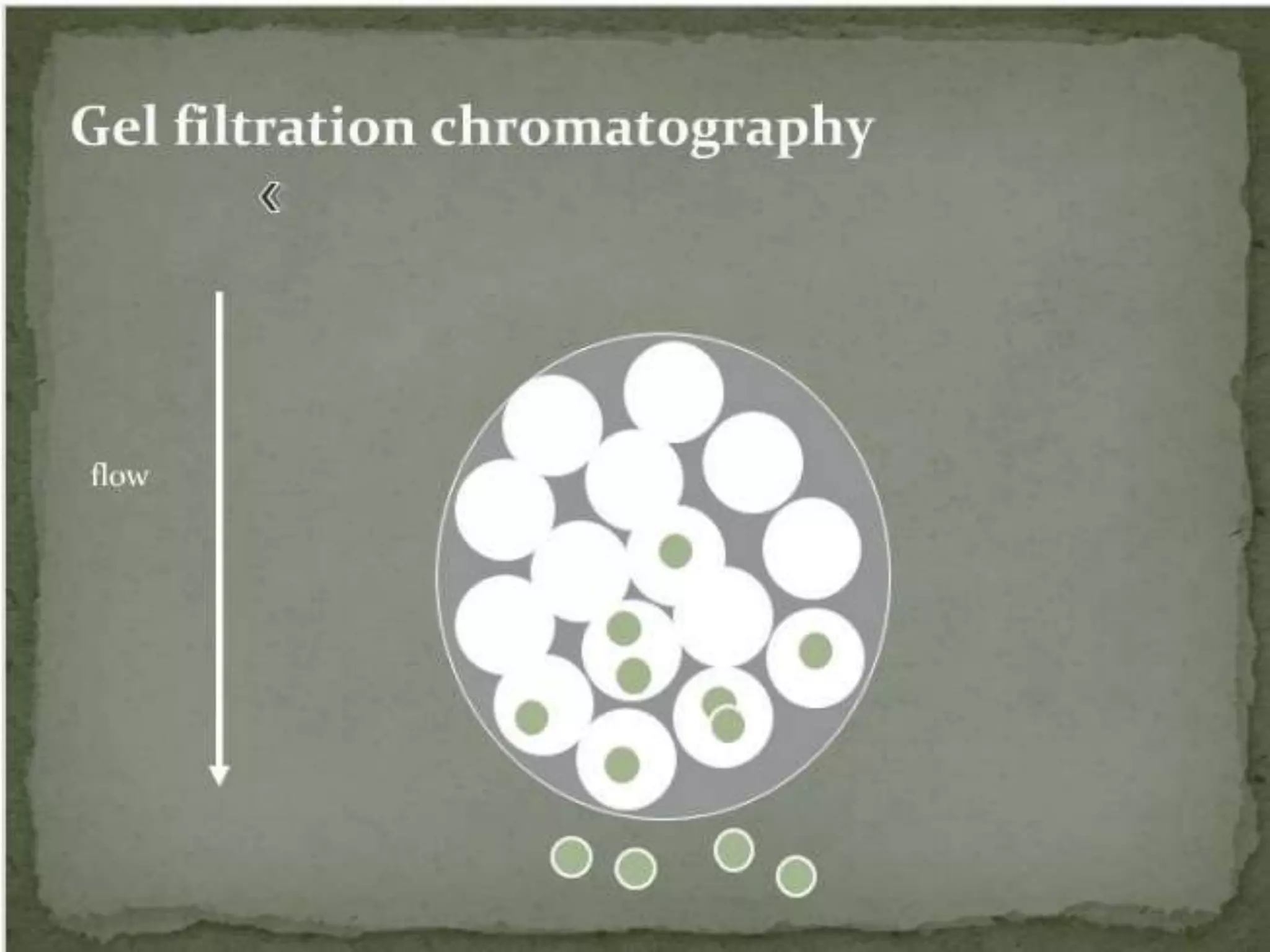
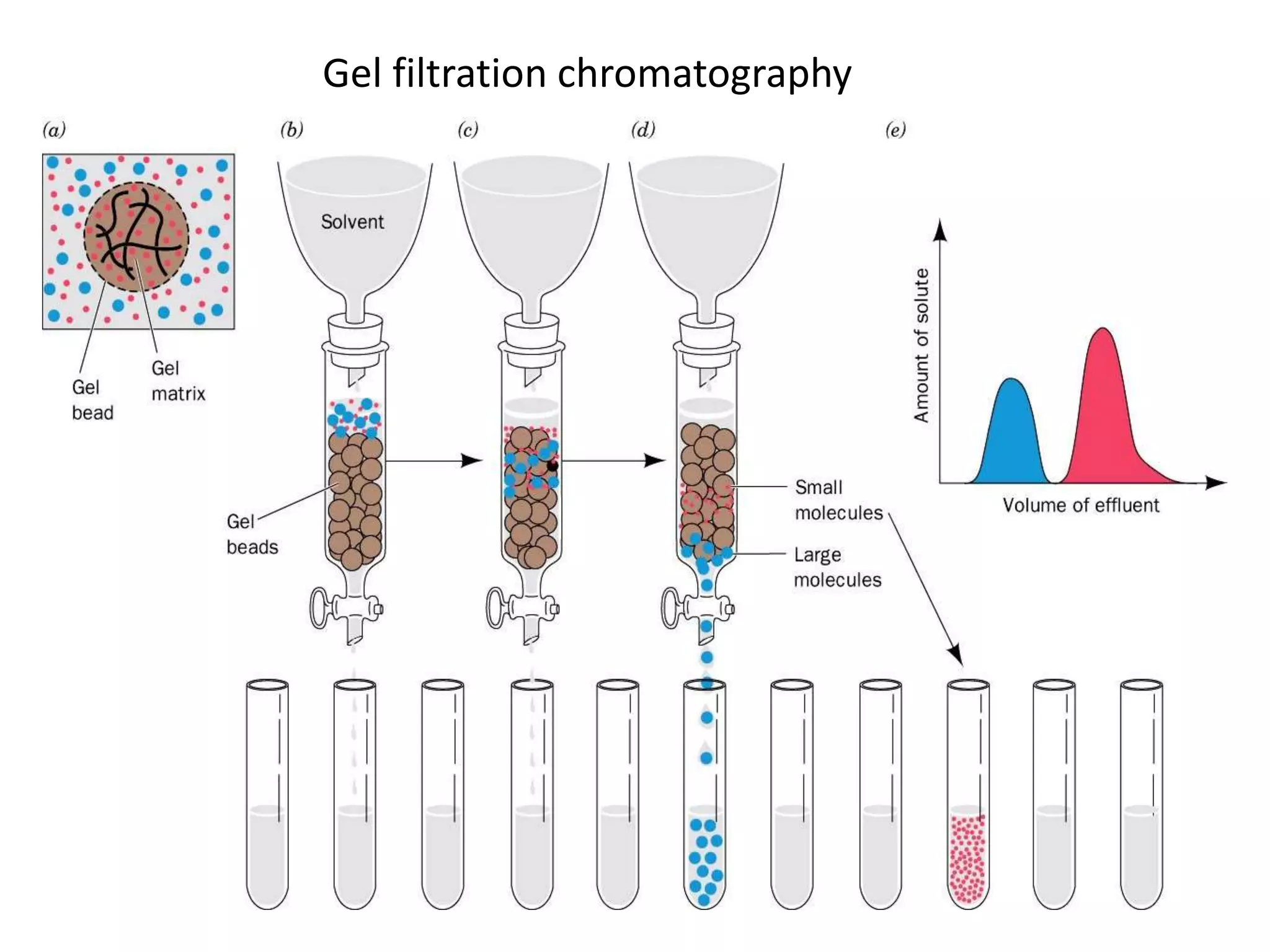
Gel filtration chromatography separates molecules based on their size. The stationary phase consists of a gel with porous beads. Smaller molecules are able to enter the pores and thus elute later, while larger molecules are excluded and elute faster. Common gels used include dextran (Sephadex), agarose, and acrylamide. Gel filtration chromatography can be used to separate proteins, determine molecular weights, and desalt macromolecules.