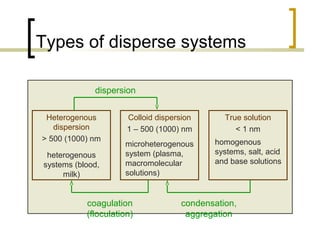
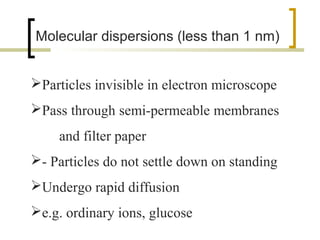
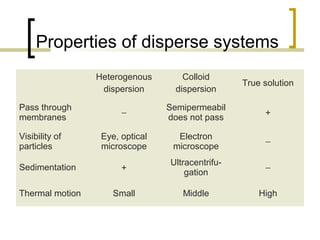
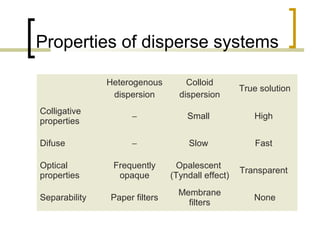
This document discusses disperse systems, which are mixtures where one substance is dispersed throughout another. It defines three main types of disperse systems: true solutions (particles <1 nm), colloidal dispersions (particles 1 nm to 500 nm), and heterogeneous dispersions (particles >500 nm). It provides characteristics of each type, such as visibility of particles, ability to pass through filters or membranes, sedimentation rates, and thermal motion of particles. The document also classifies disperse systems based on the state of the dispersed and continuous phases (gas, liquid, solid) and lists some key properties like colligative effects, diffusion rates, optical properties, and methods of separation.