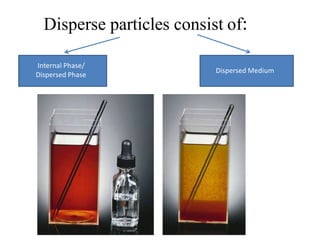
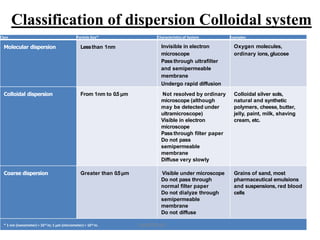
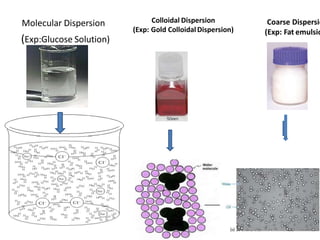
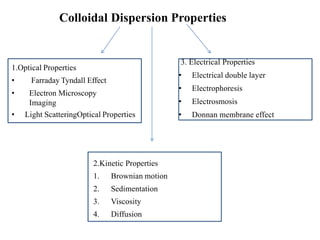
1. A colloidal dispersion system consists of dispersed particles uniformly distributed in a dispersion medium. The particle size ranges from 1nm to 1 micron.
2. Examples of colloidal systems include liposomes, nanoparticles, paint, milk, and radioactive colloids. They pass through filter paper but not semipermeable membranes and diffuse very slowly.
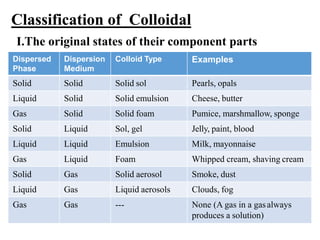
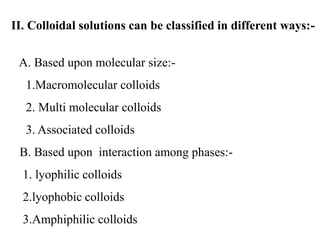
3. Colloidal dispersions can be classified based on particle size, state of the dispersed and continuous phases, and interaction between phases. Lyophilic colloids readily interact with the dispersion medium while lyophobic colloids have little attraction. Amphiphilic colloids are composed of surface-active agents that form micelles above