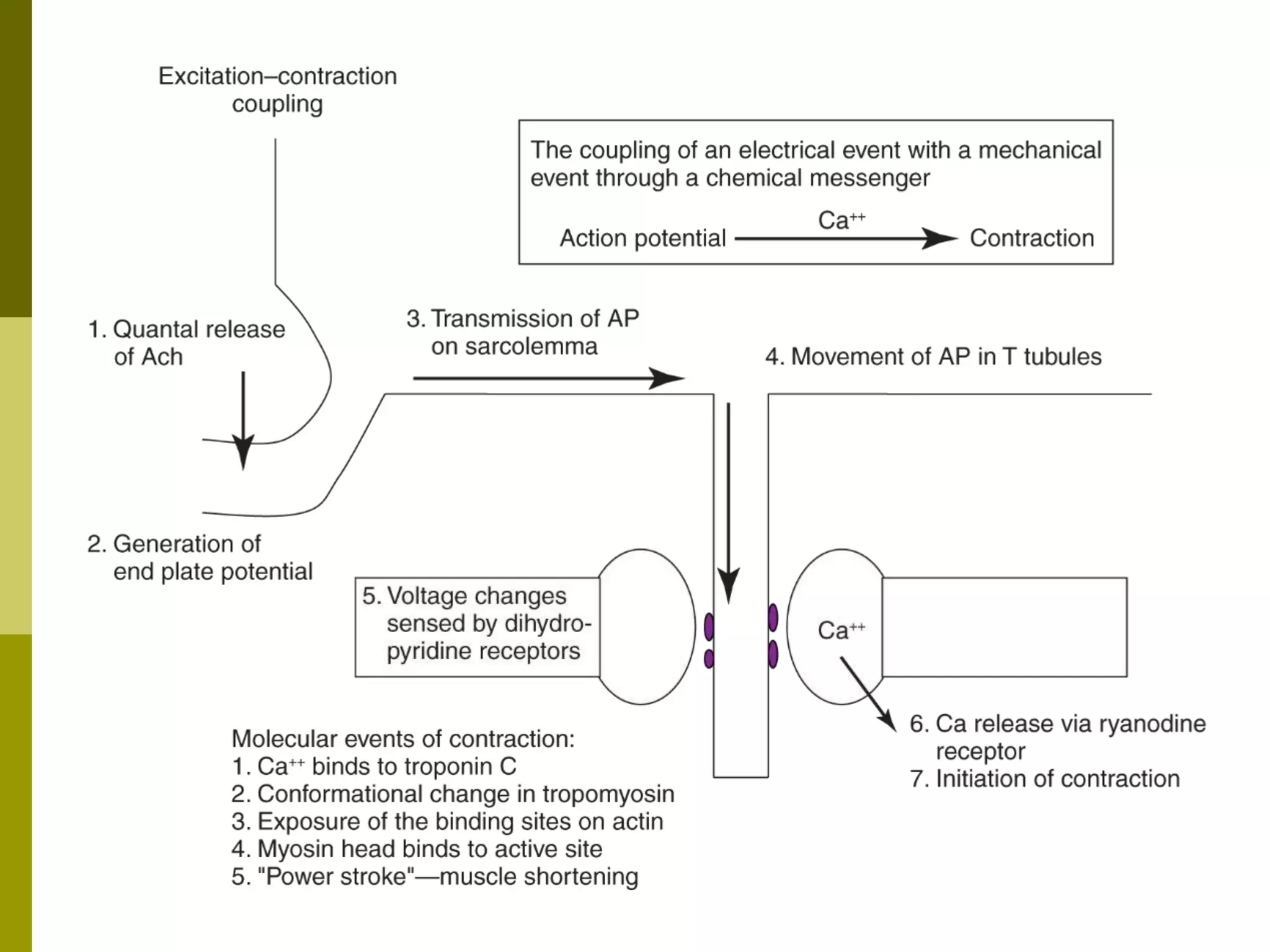
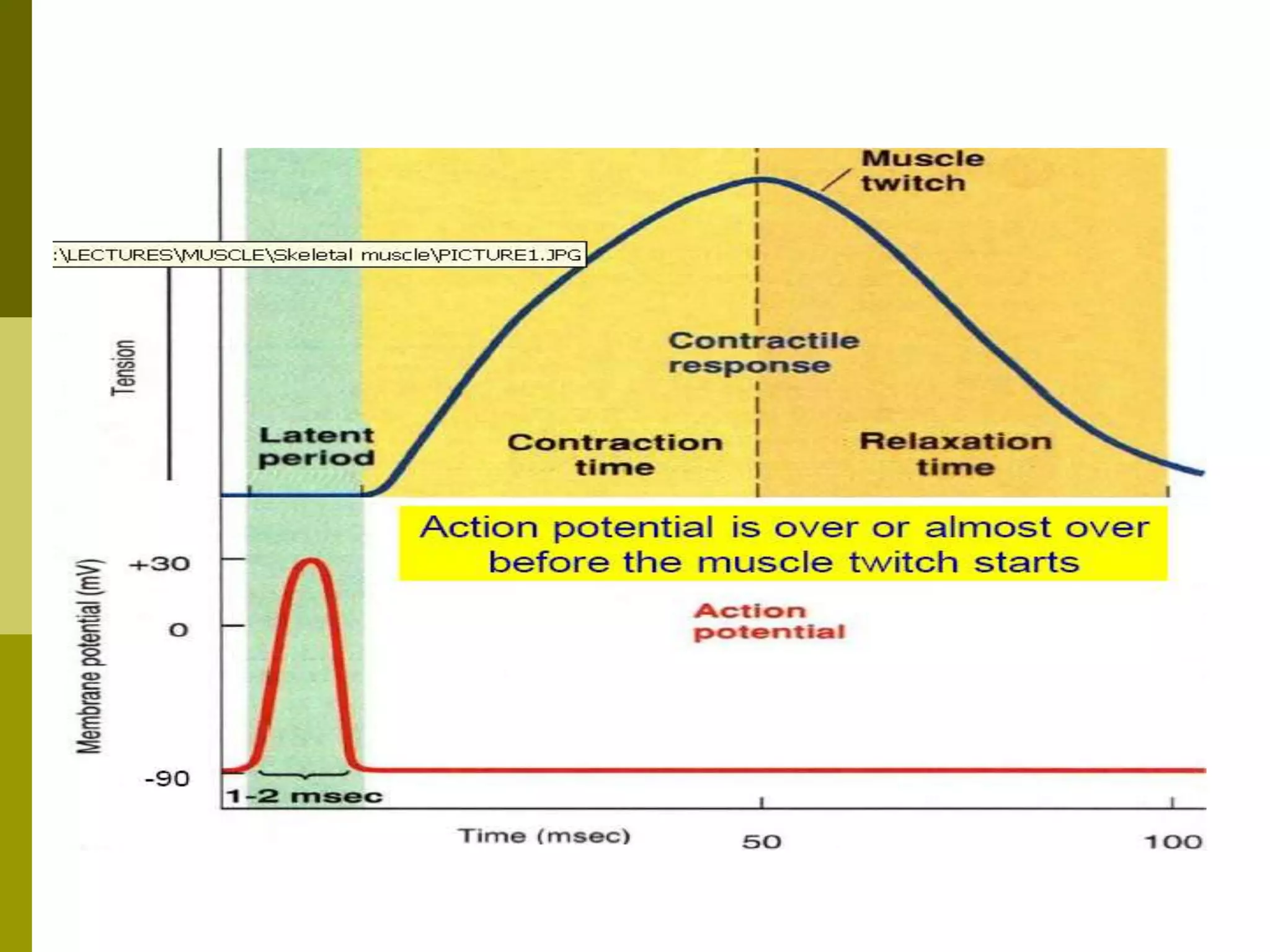
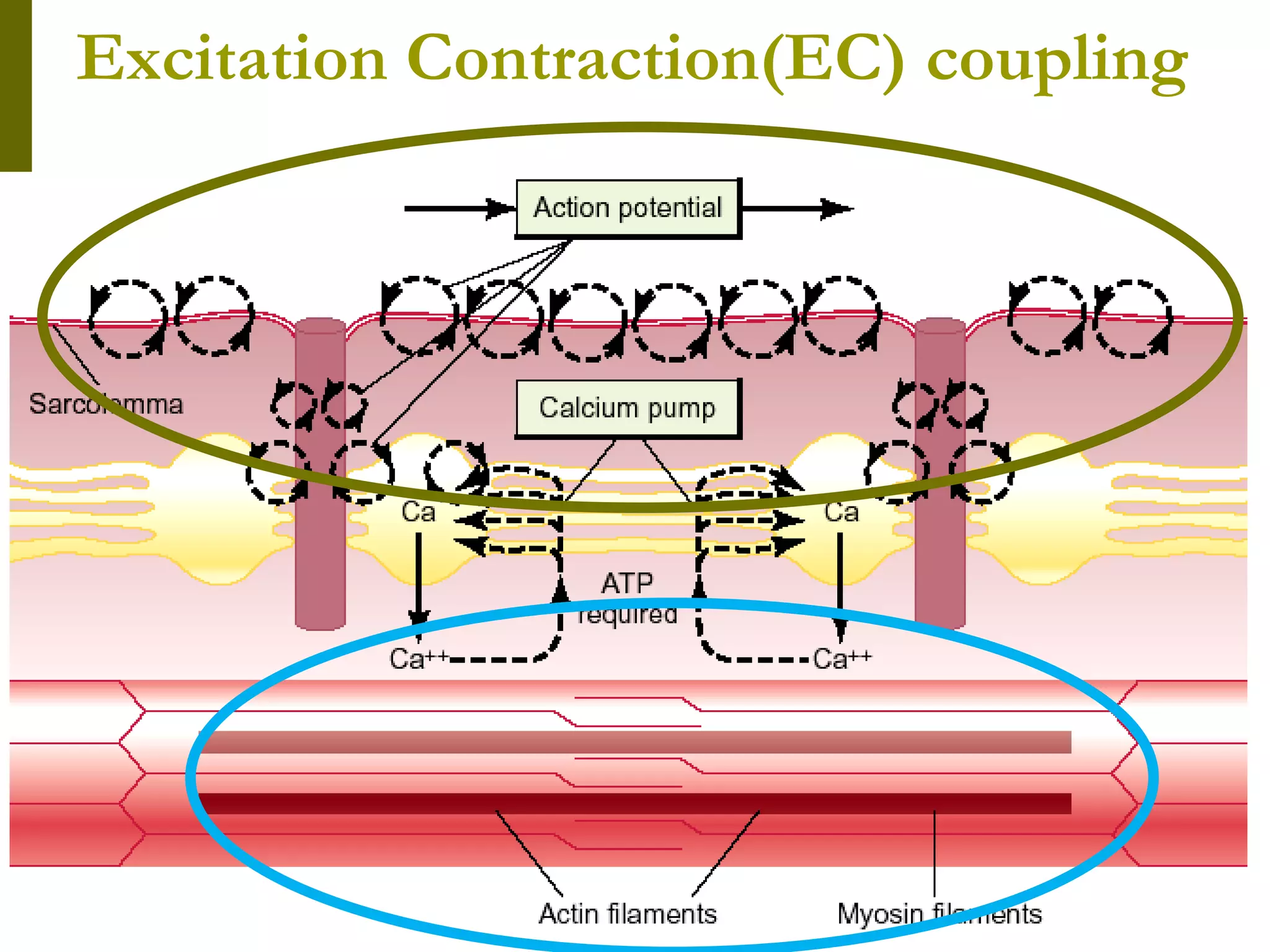
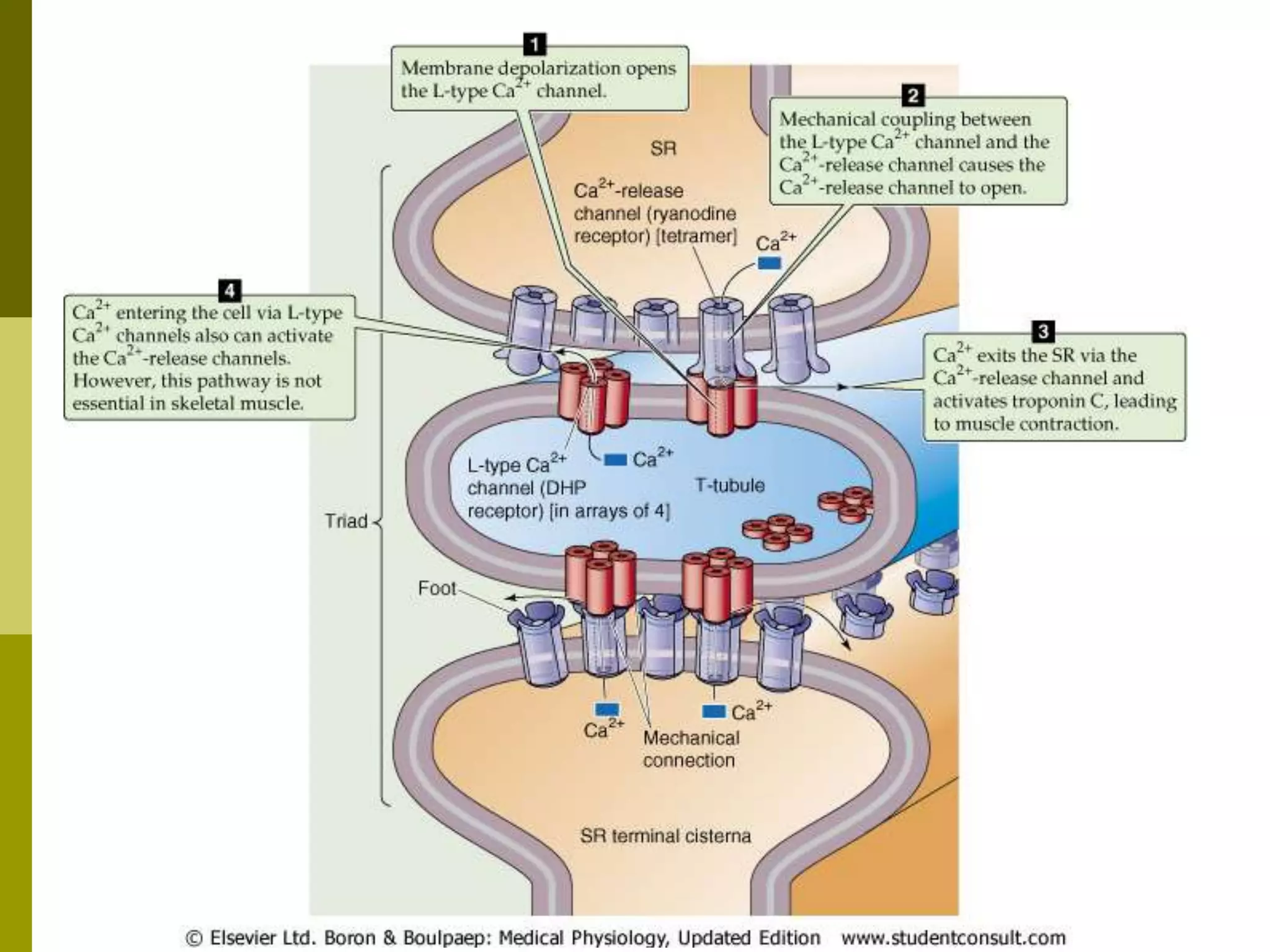
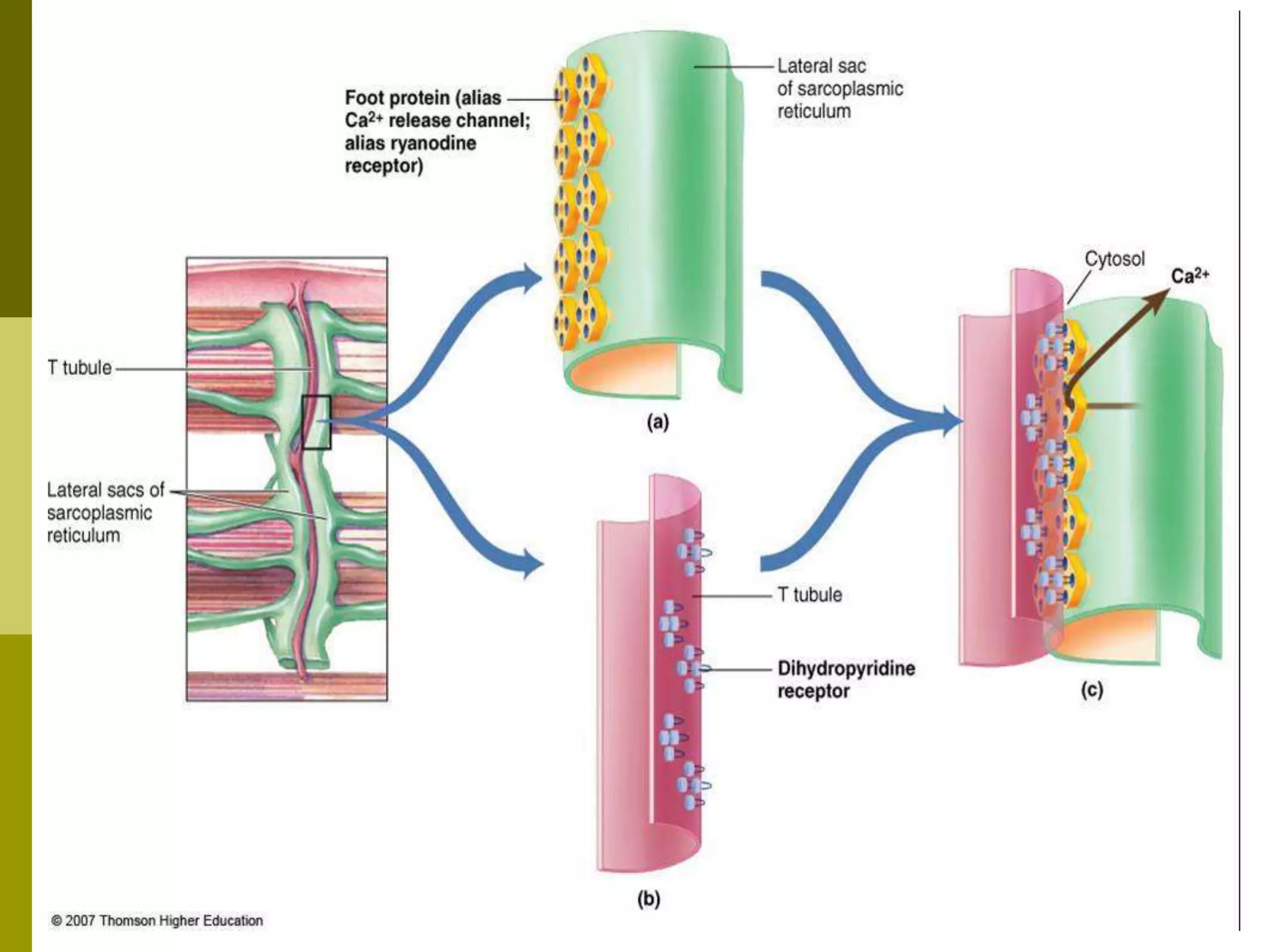
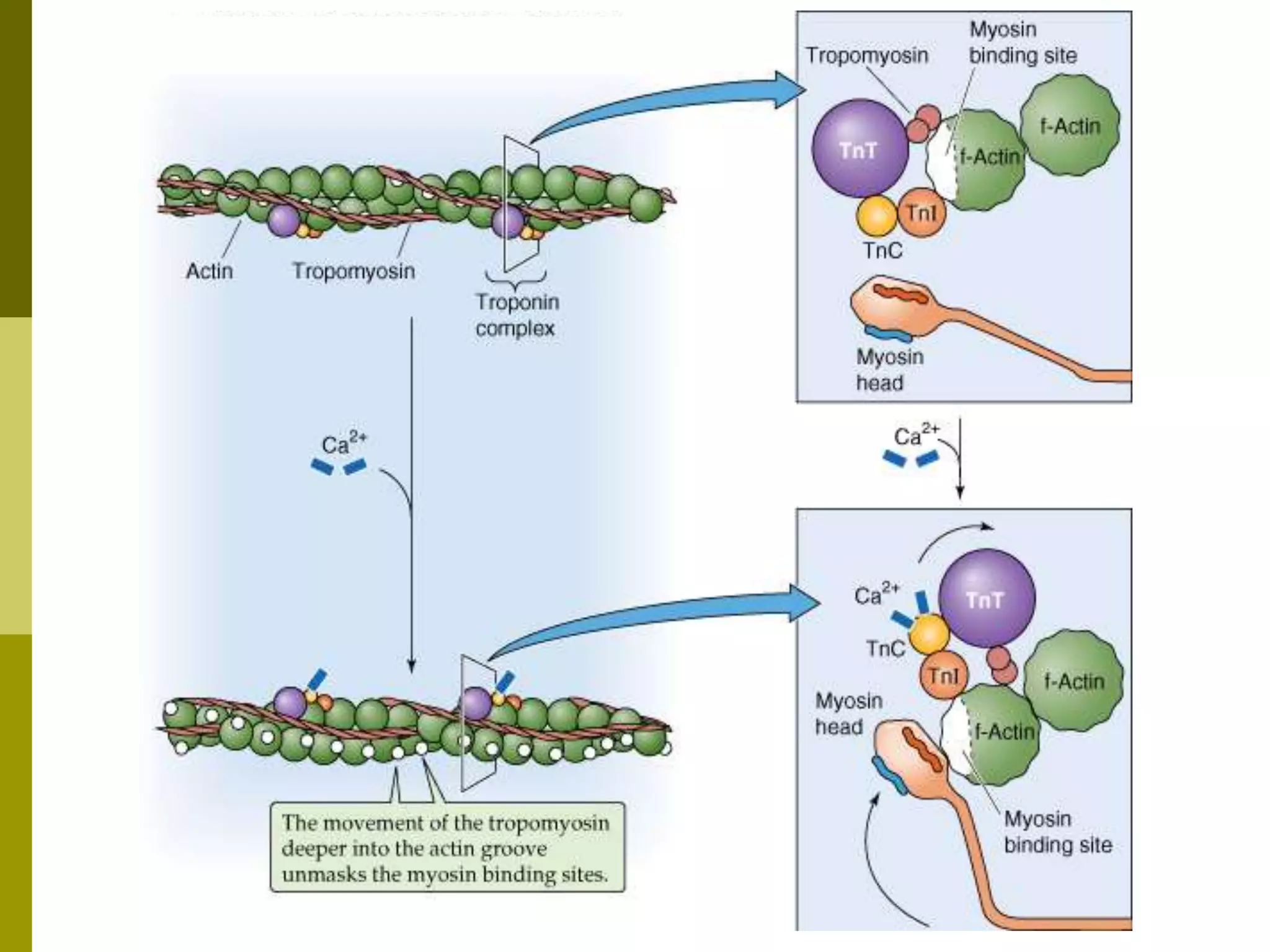
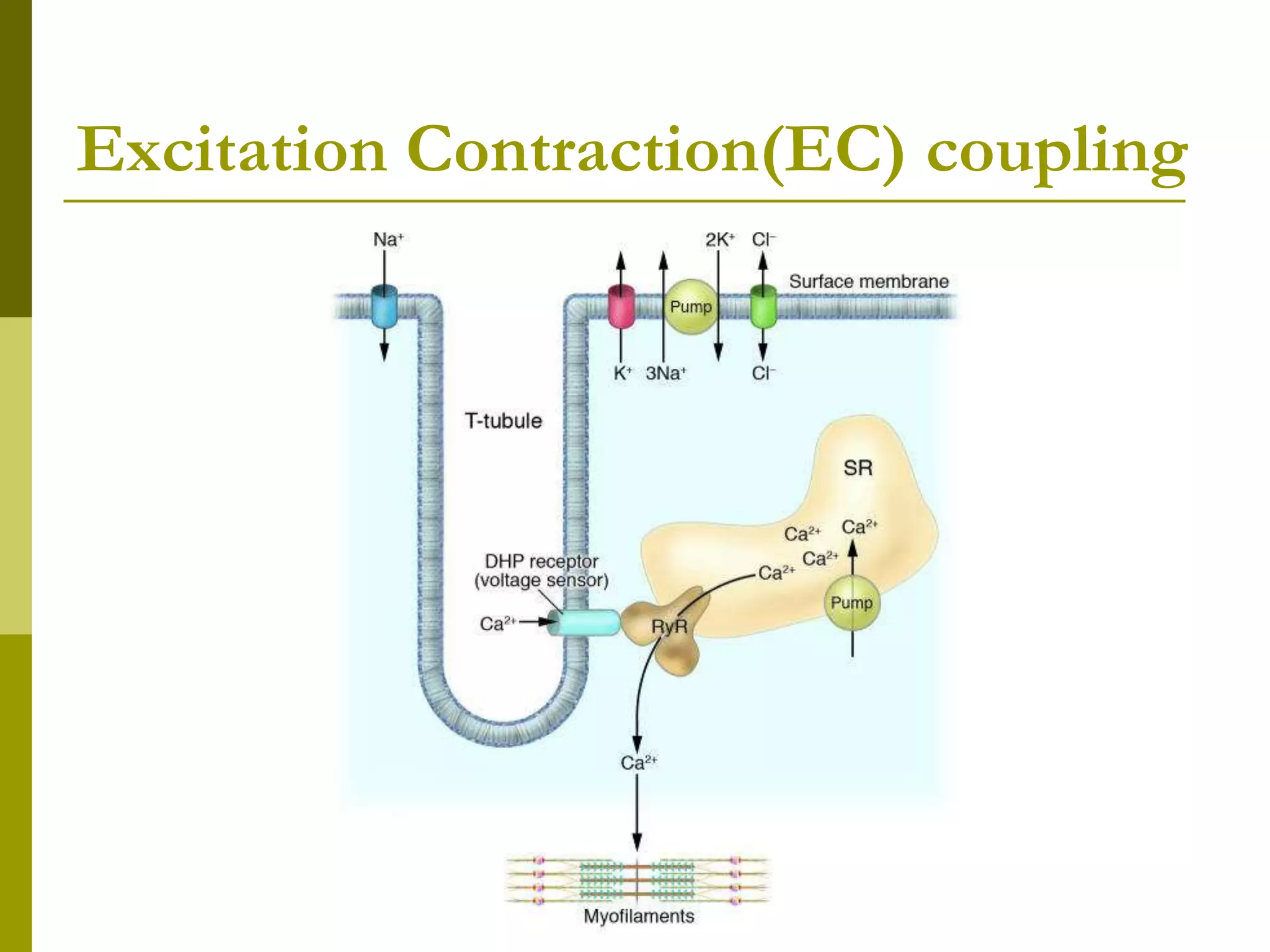
The document summarizes the mechanism of skeletal muscle contraction. It describes how an action potential leads to a rise in intracellular calcium levels through excitation-contraction coupling. This triggers the sliding filament theory where actin and myosin filaments slide past each other through cross-bridge cycling powered by ATP hydrolysis. Calcium binds to troponin C, allowing the power stroke to occur as myosin heads pull the actin filaments towards the center of the sarcomere. Relaxation occurs as calcium is re-sequestered in the sarcoplasmic reticulum, breaking the cross-bridges.

























![Cross Bridge Cycle
Cycle repeats as long as Ca2+ is elevated
and sufficient ATP is there
Muscle cells do not regulate cross-bridge
cycling by modifying [ATP]i
Instead, skeletal muscle and cardiac
muscle control this cycle by preventing
cross-bridge formation until the
tropomyosin moves out of the way in
response to an increase in [Ca2+]i](https://image.slidesharecdn.com/muscle3-excitationcontractioncoupling-180414105857/75/Excitation-Contraction-coupling-34-2048.jpg)








![Steps in Relaxation
Cell may extrude Ca2+ using either an Na-
Ca exchanger (NCX) or a Ca2+ pump(PMCA)
However, would eventually deplete the cell of
Ca2+ and is thus a minor mechanism for Ca2+
removal from the cytoplasm
Instead, Ca2+ re-uptake into the SR is the
most important mechanism by which the
cell returns [Ca2+]i to resting levels
Ca2+ re-uptake by the SR is mediated by a
SERCA(s arcoplasmic or e ndoplasmic
r eticulum C a2+A TPase )-type Ca2+ pump](https://image.slidesharecdn.com/muscle3-excitationcontractioncoupling-180414105857/75/Excitation-Contraction-coupling-45-2048.jpg)
![Steps in Relaxation
SR Ca2+-pump activity is inhibited by high [Ca2+]
within the SR lumen
Inhibition of SR Ca2+-pump activity is delayed by
Ca2+-binding proteins within the SR lumen
Buffer the Ca2+ increase in the SR during Ca2+ re-uptake
and thus markedly increase the Ca2+ capacity of SR
Proteins have a tremendous capacity to bind Ca2+ with
up to 50 binding sites per protein molecule
Principal Ca2+ binding protein in skeletal muscle,
calsequestrin
also present in cardiac and some smooth muscle
Calreticulin - Ca2+-binding protein found in particularly
high concentrations within the SR of smooth muscle](https://image.slidesharecdn.com/muscle3-excitationcontractioncoupling-180414105857/75/Excitation-Contraction-coupling-46-2048.jpg)