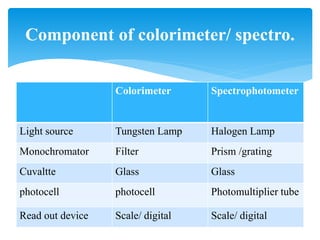
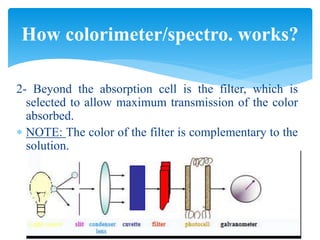
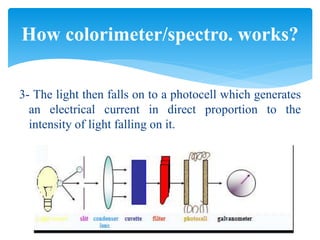
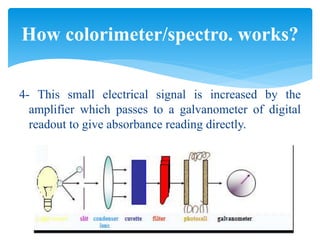
This document discusses colorimetry and spectrophotometry techniques. It explains that Beer's law and Lambert's law describe how the absorption of light by a colored solution depends on concentration and path length. A colorimeter uses a light source and filter to measure absorption, while a spectrophotometer uses a prism or grating to provide a narrow range of wavelengths, allowing distinction between closely related absorptions. Common applications include determination of substances in biological samples.