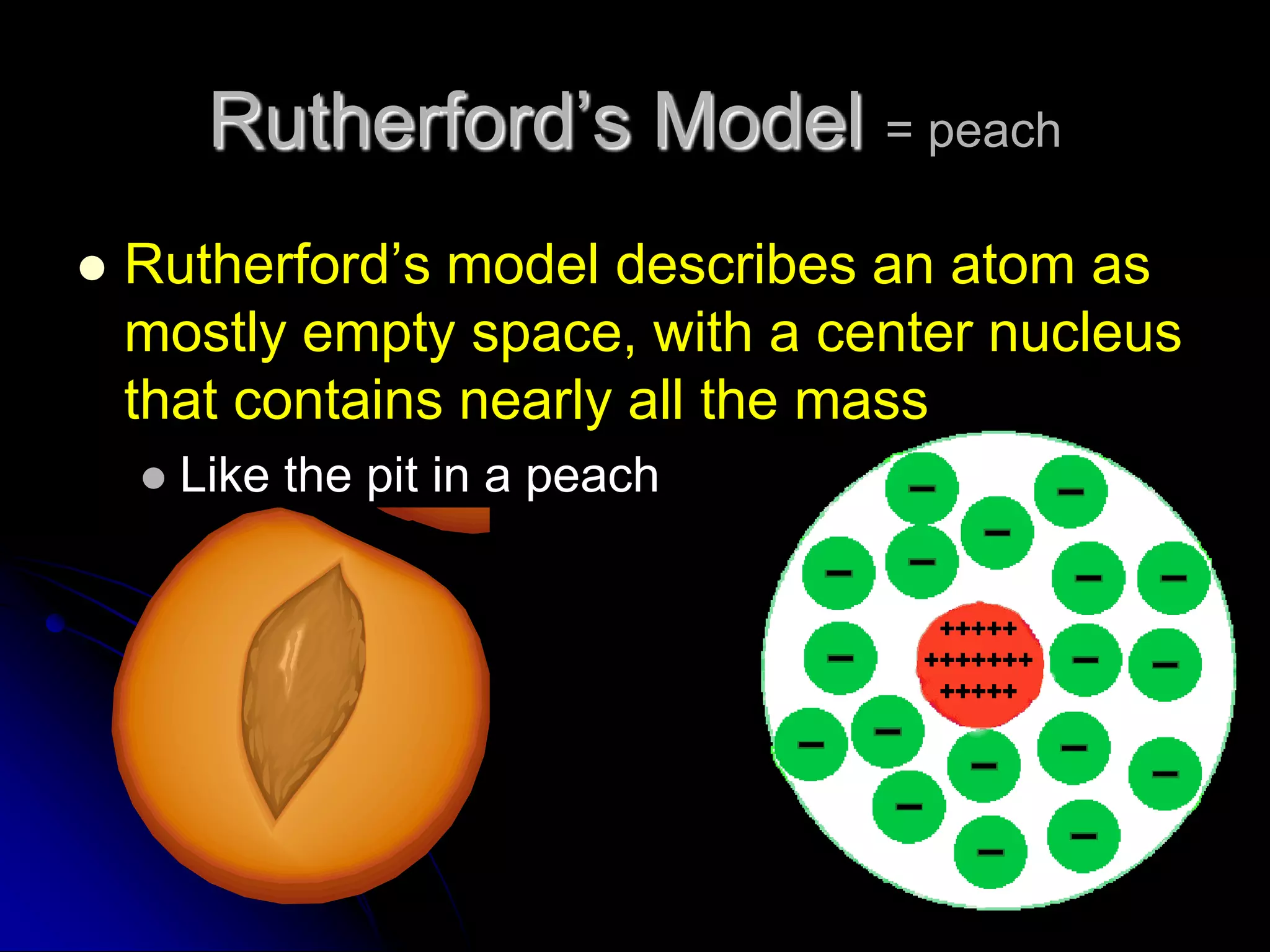
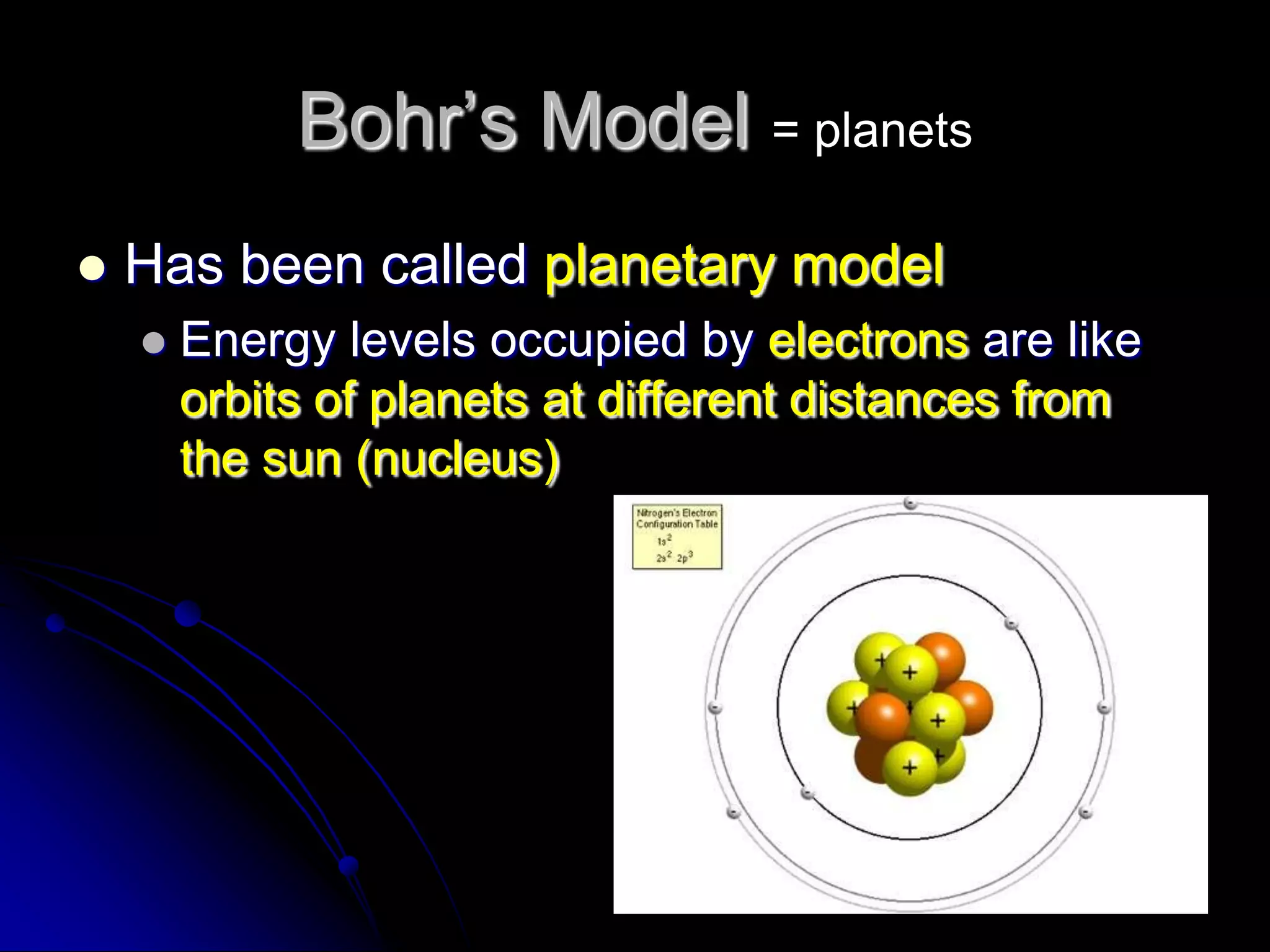
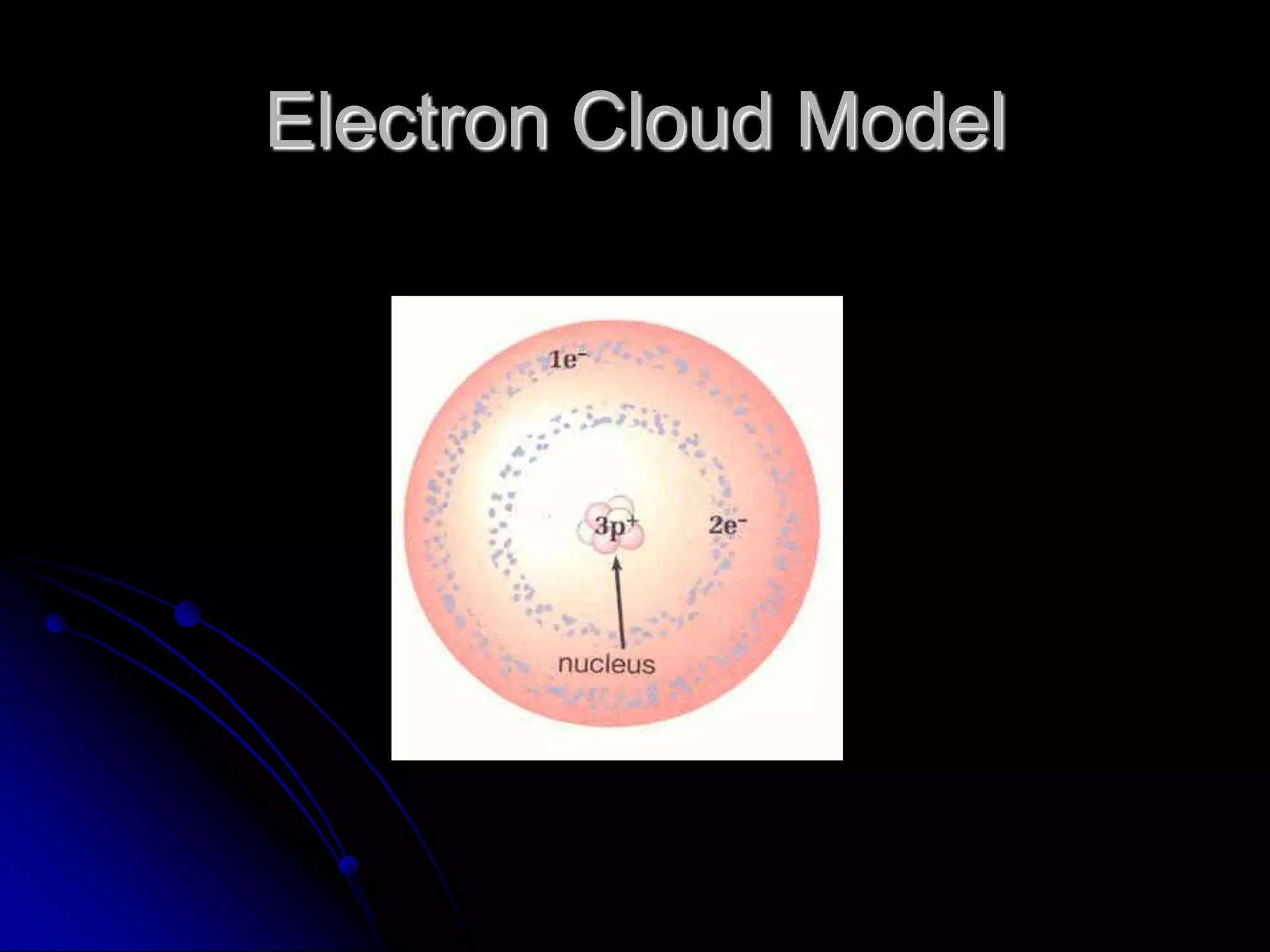
The document summarizes the development of atomic theory over thousands of years through the proposals of different atomic models by scientists like Democritus, Dalton, Thomson, Rutherford, Bohr, and others. It describes how atomic models have evolved from early concepts of atoms as indivisible spheres to the current understanding of atoms having a small, dense nucleus surrounded by an electron cloud.