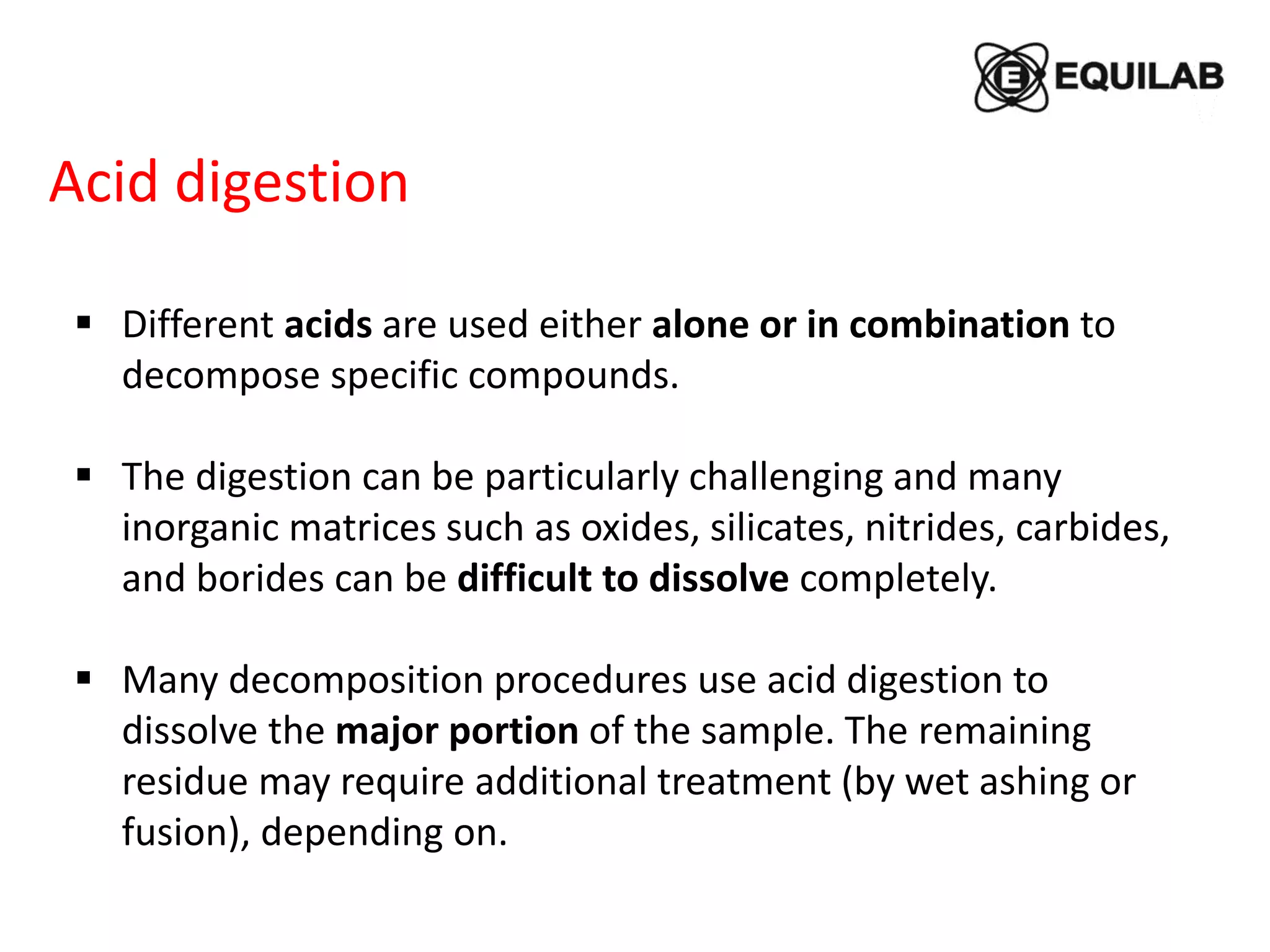
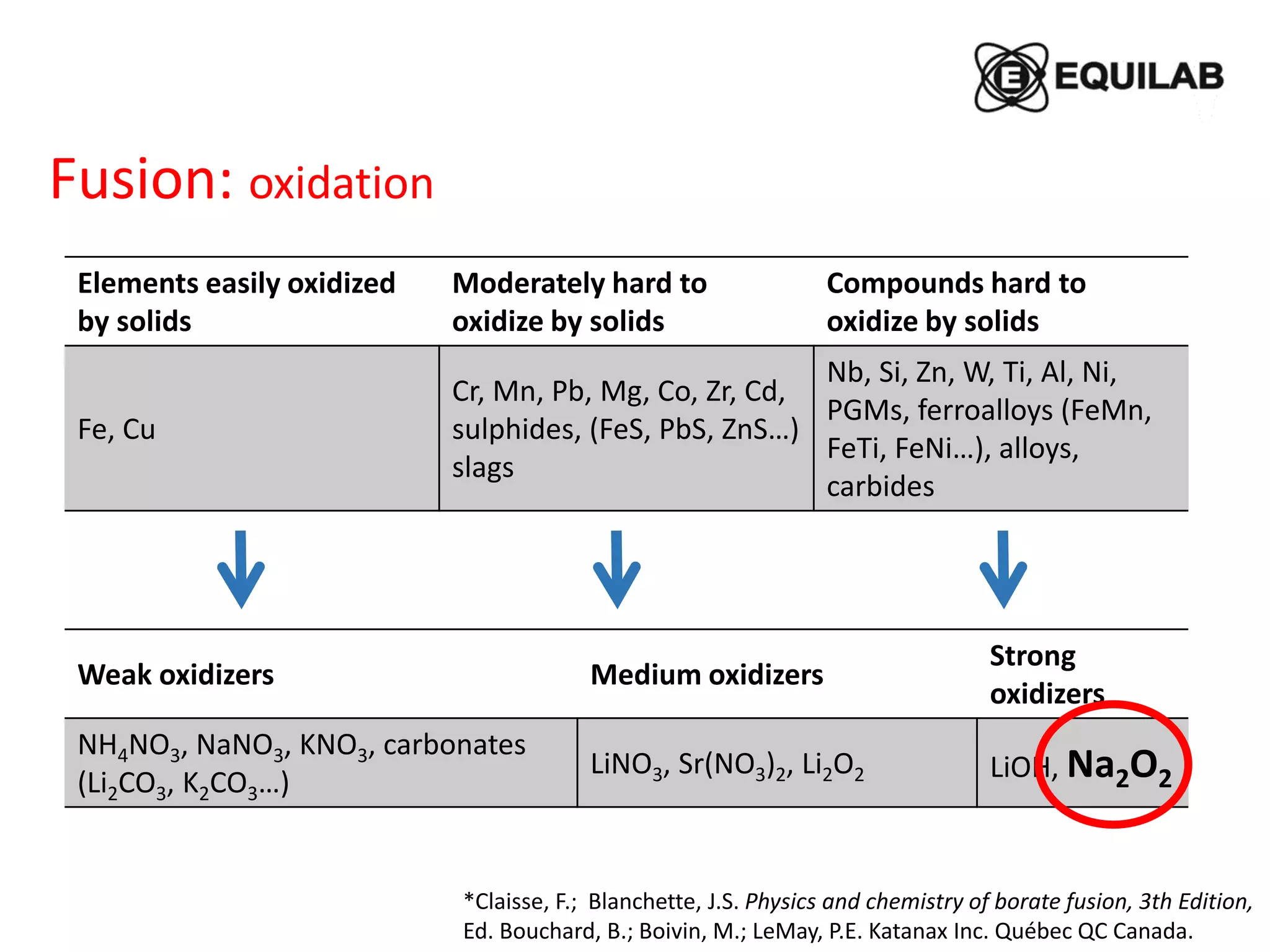
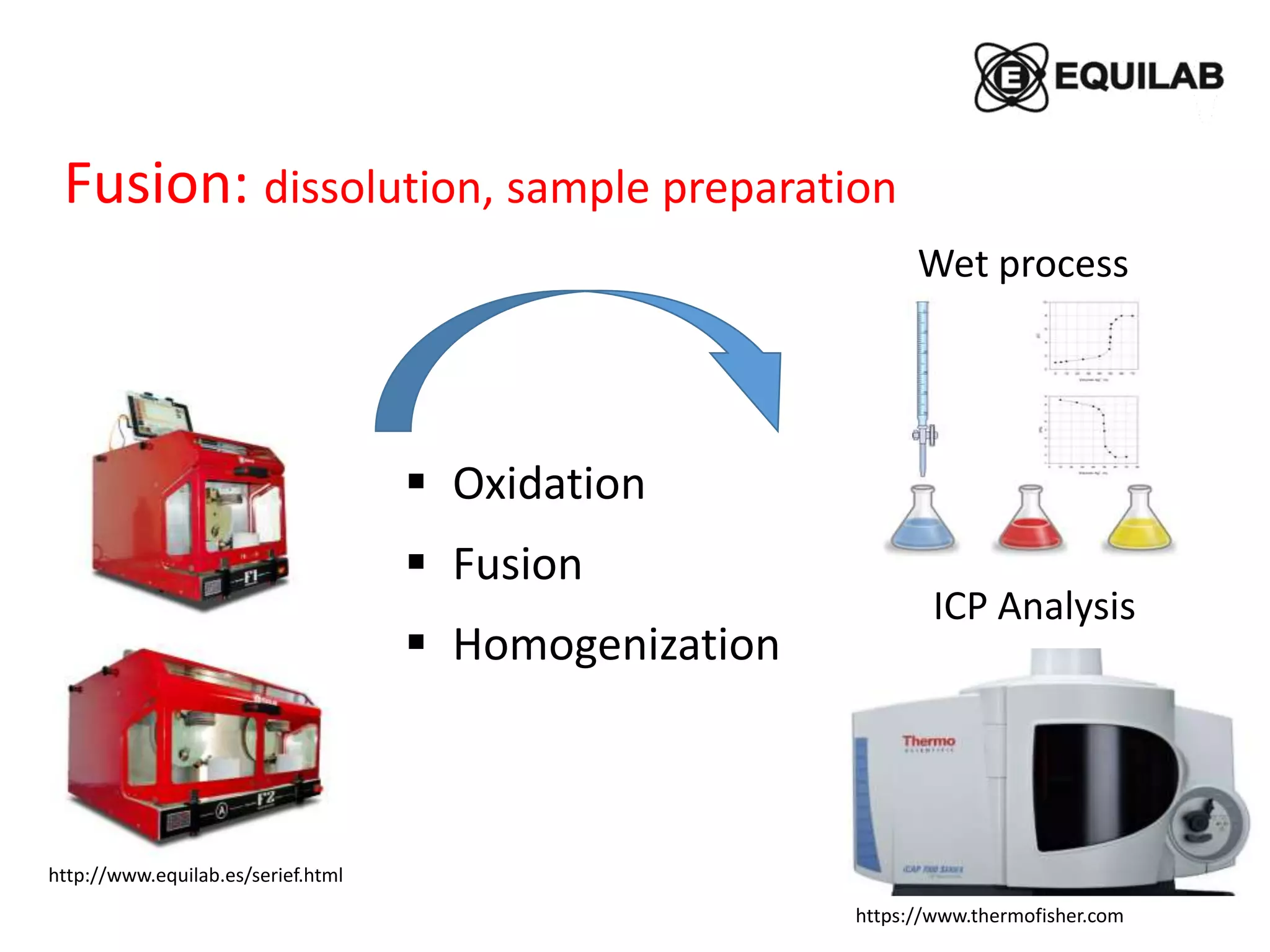
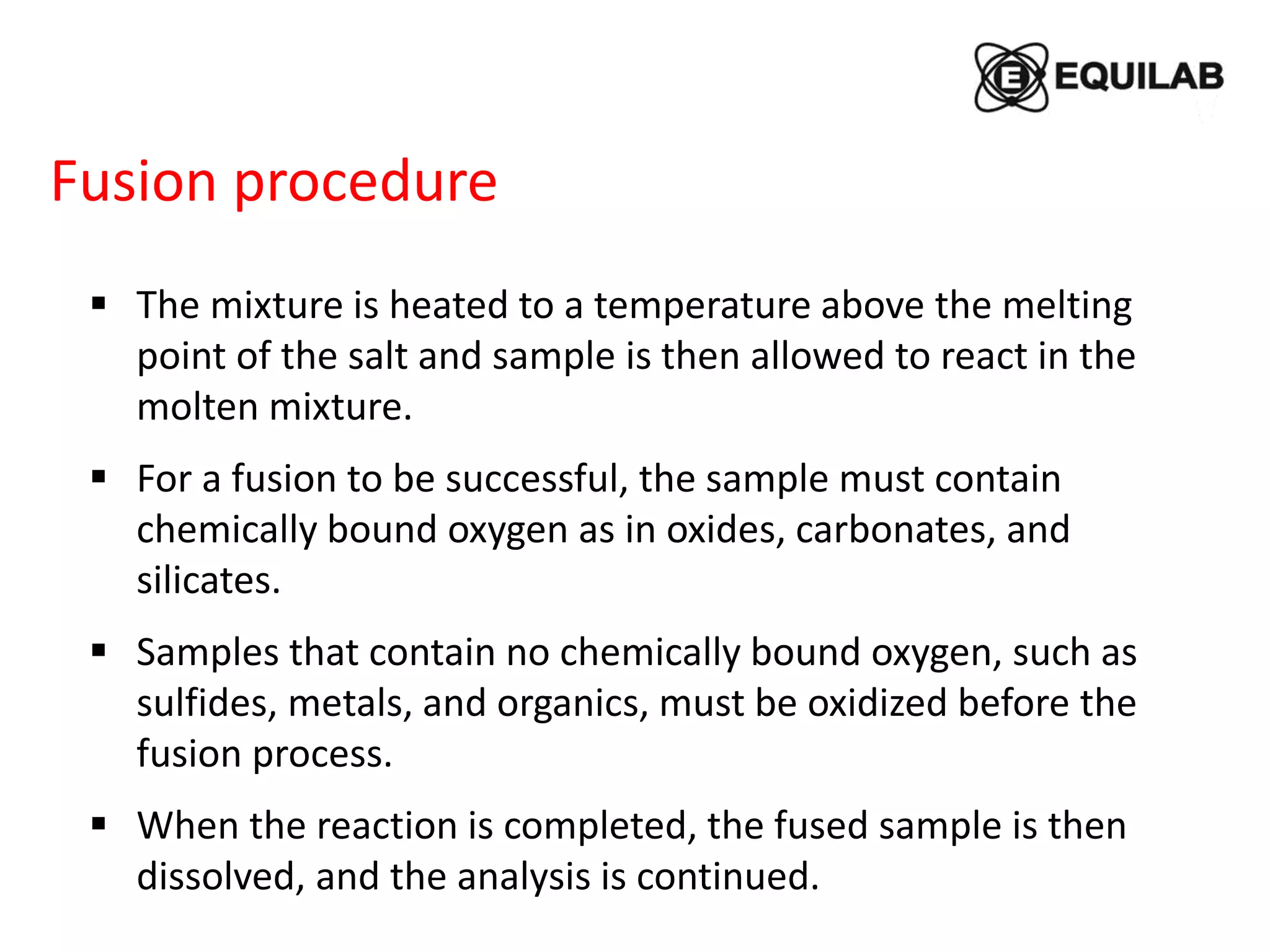
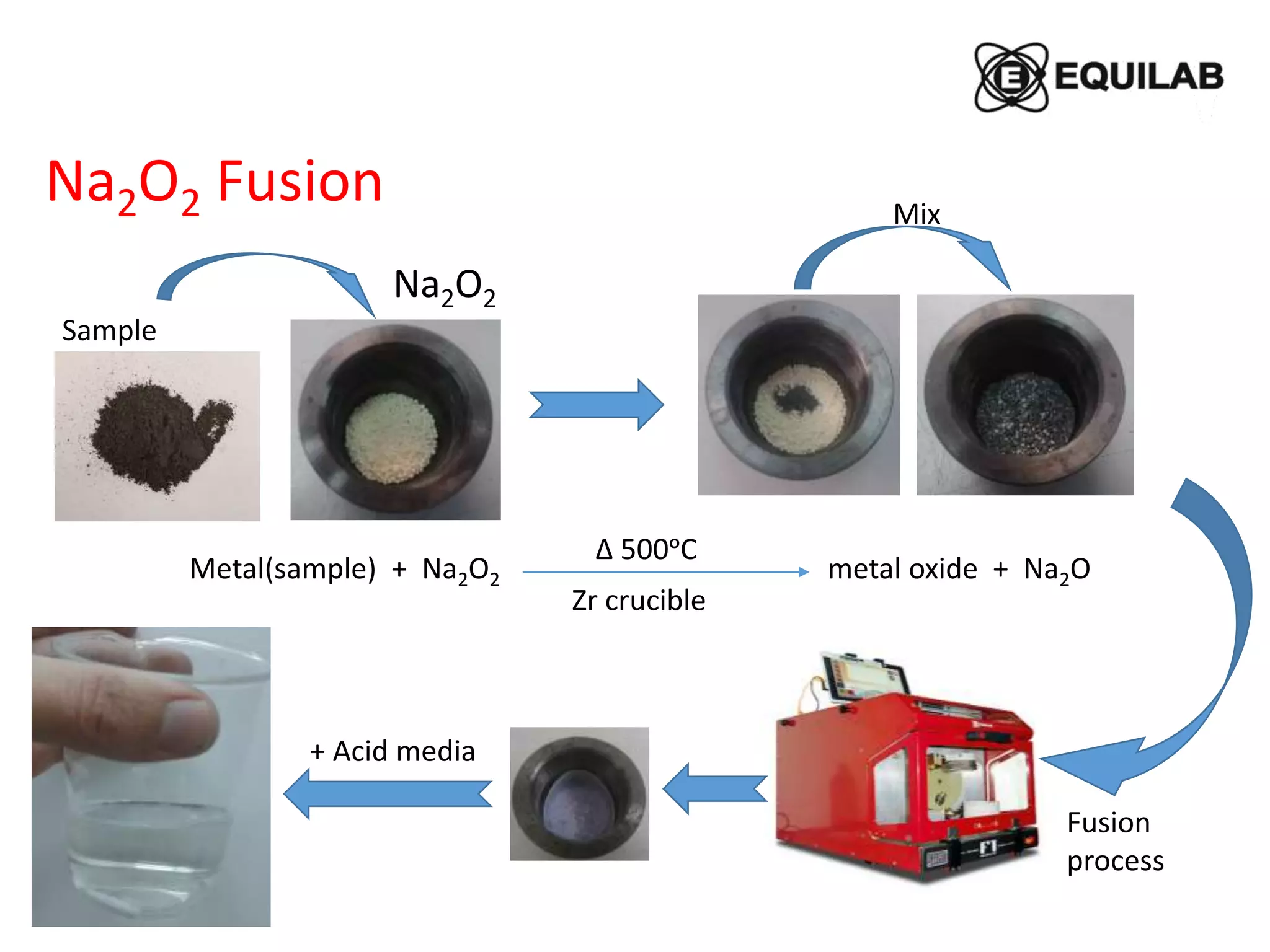
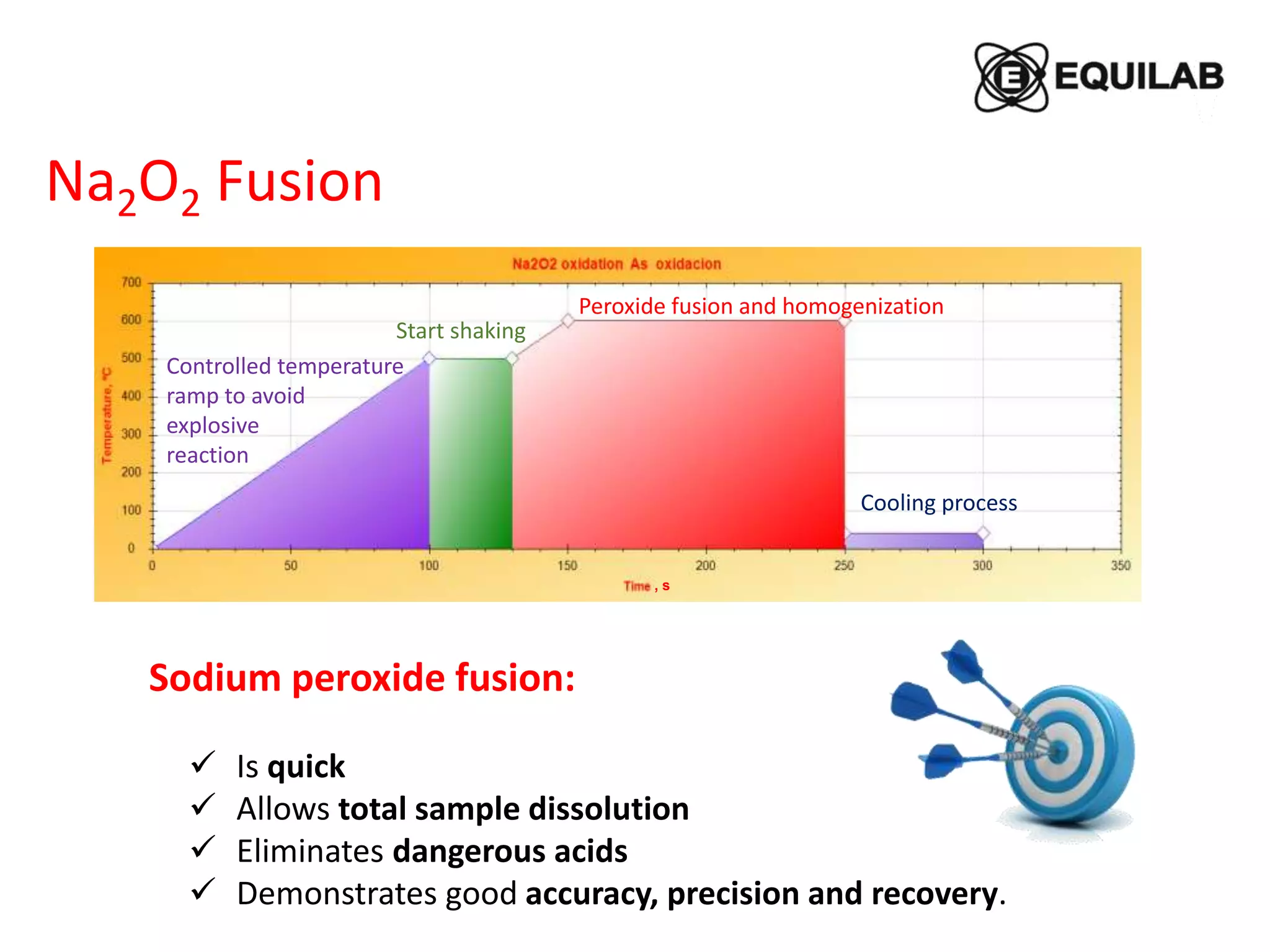
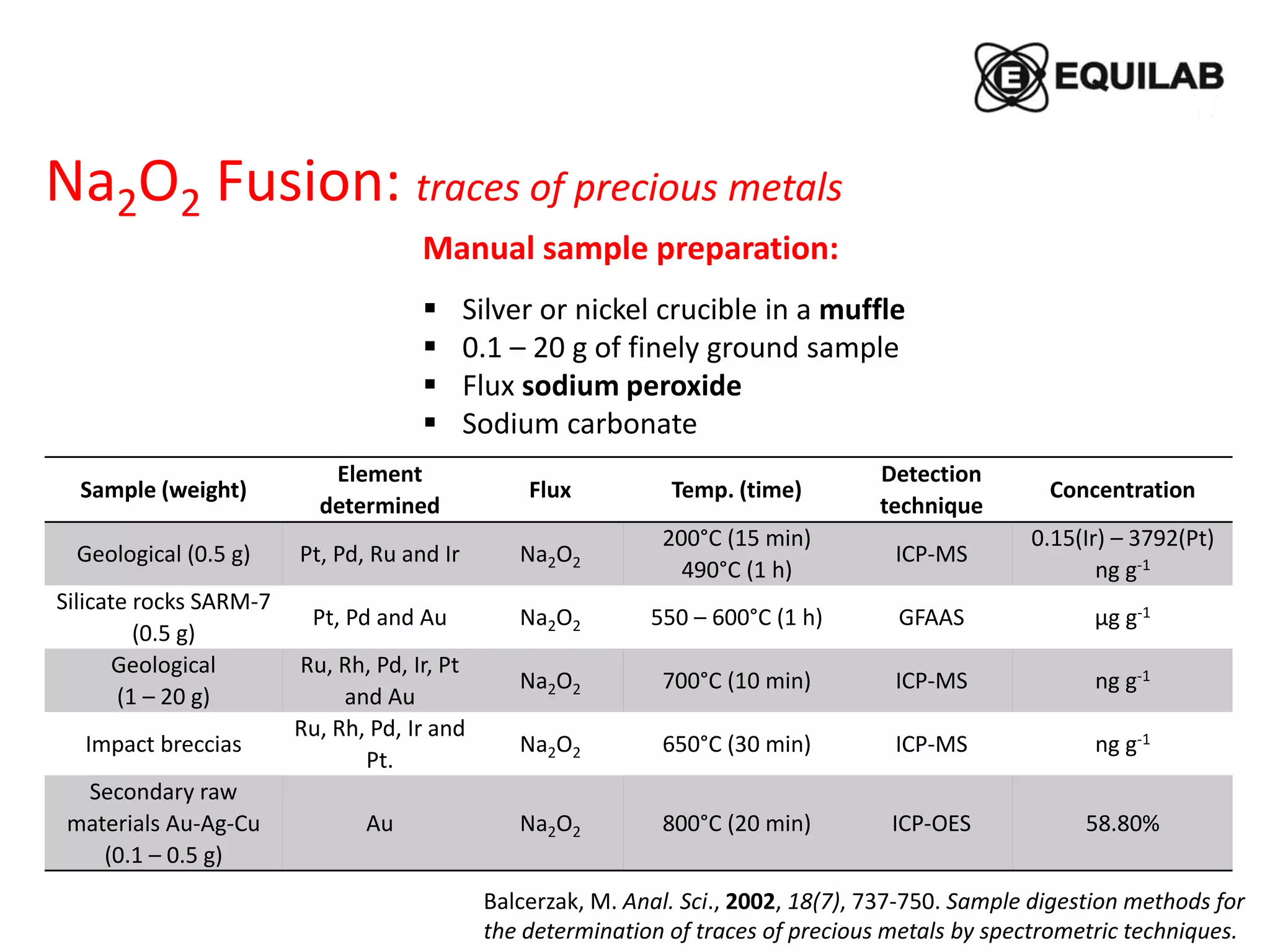
Sodium peroxide fusion is an effective sample dissolution technique that provides complete digestion of samples in a short period of time. It avoids the use of dangerous acids and allows for accurate, precise, and reproducible analysis by ICP-OES and ICP-MS. The process involves mixing the sample with sodium peroxide flux in a crucible, heating to melt and fuse the mixture, then dissolving the cooled fused bead in acid for elemental analysis. Sodium peroxide fusion has been shown to quantitatively dissolve a variety of materials like minerals, alloys, and precious metals samples.







![Na2O2 Fusion: Rare earth elements by ICP-OES and ICP-MS
Application note, 2018. Analysis of Rare Earth Elements by ICP-OES and ICP-MS Potentials and Limitations.
Analytik Jena AG, an Endress+Hauser Company. Jena Germany. www.analytik-jena.com
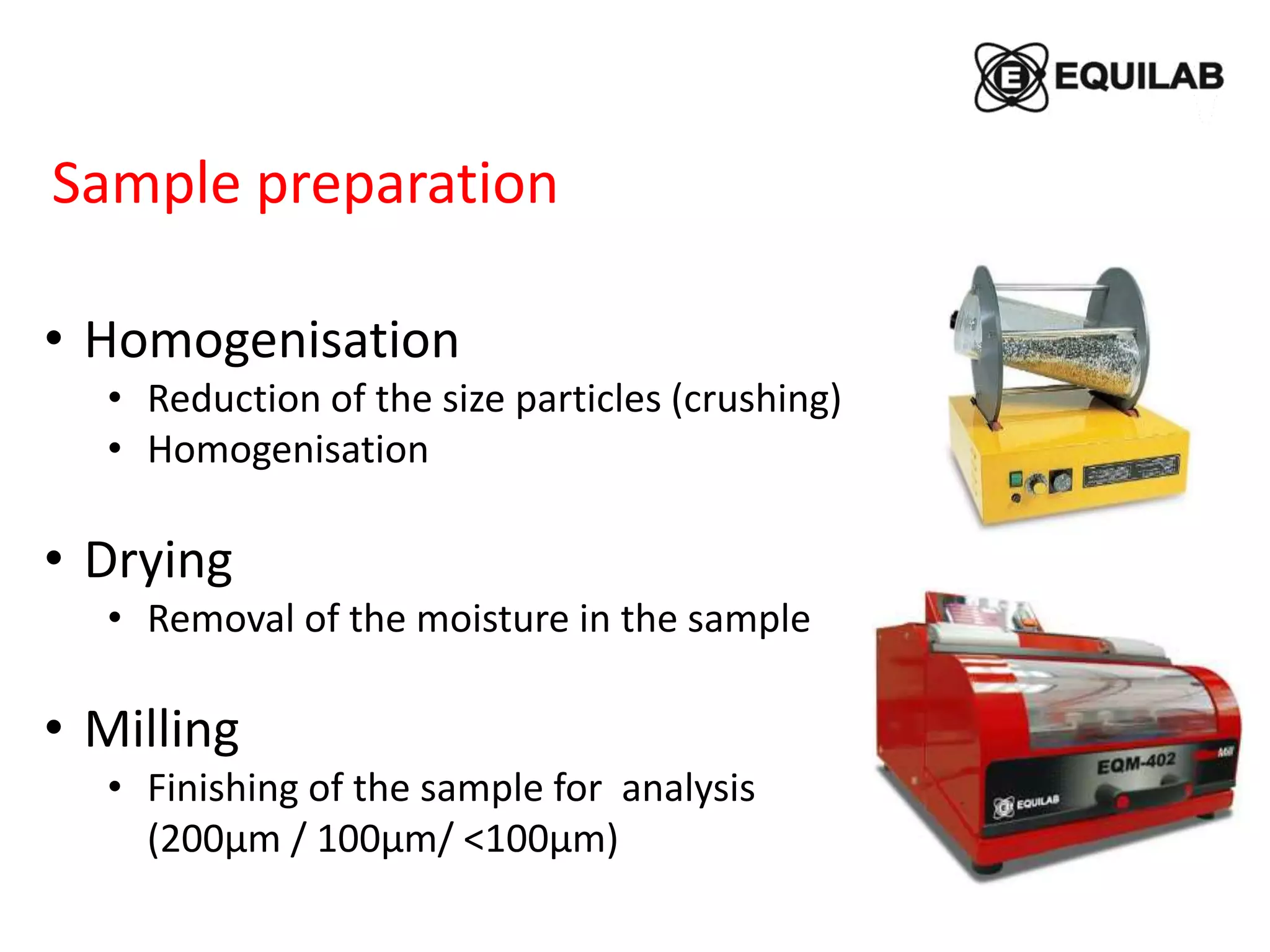
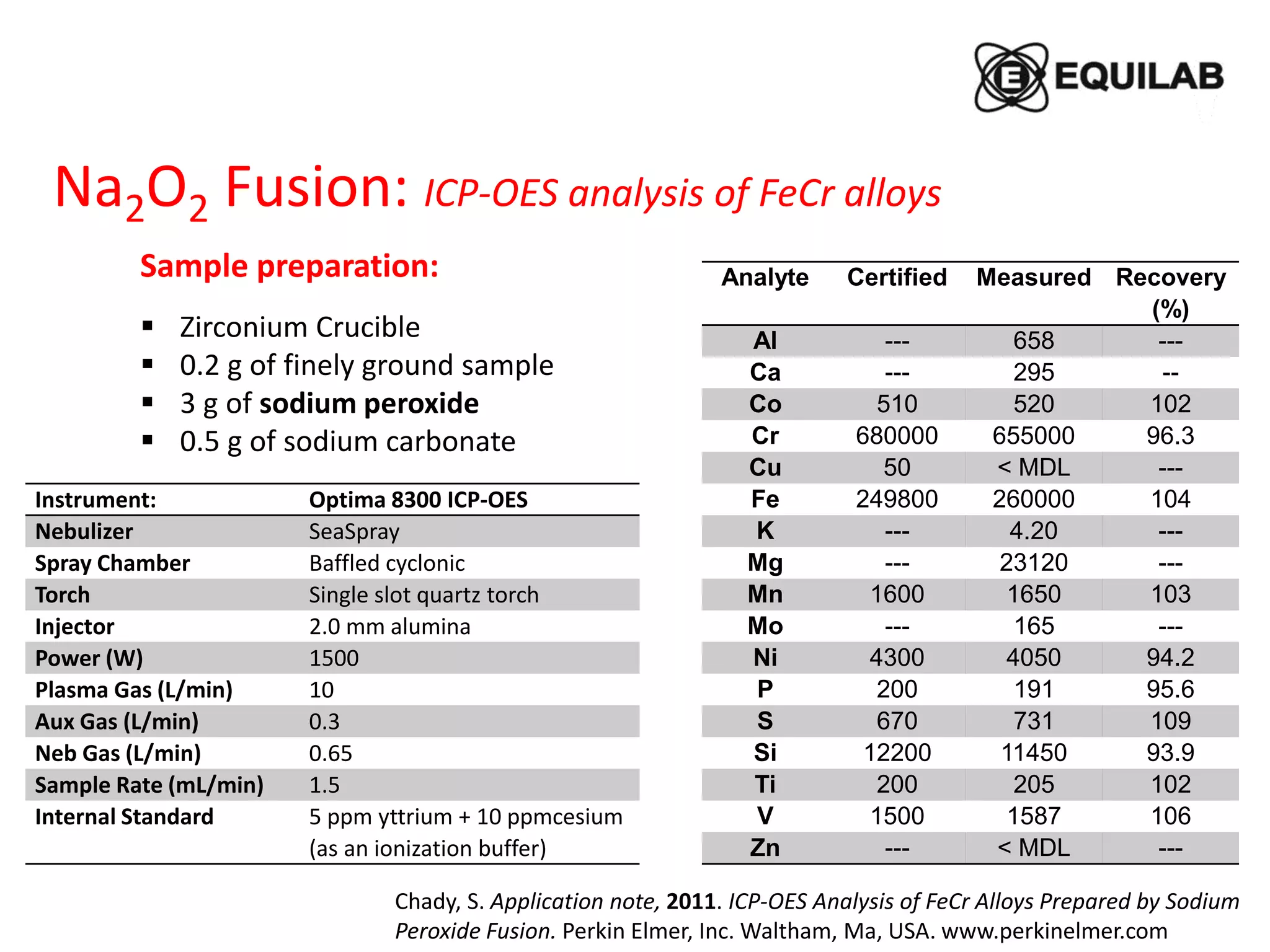
Sample preparation:
Porcelain crucible (30 x 30 mm) in a muffle
100 mg of ground sample 200- mesh
600 mg sodium peroxide
480 ± 10ᵒC, 30 min
Dissolved in a aqueous HCl + HNO3
Element
CRM GBW 7103
(GSR-1) Granite
powder [mg/kg]
Measured
[mg/kg]
Recovery
[%]
MDL
[mg/kg]
La 54 ± 4 53.4 99 0.14
Ce 108 ± 7 112 112 0.85
Pr 12.7 ± 0.8 12.2 96 1.55
Nd 47 ± 4 48.6 106 0.34
Sm 9.7 ± 0.8 9.23 95 0.65
Eu 0.85 ± 0.07 0.71 84 0.04
Gd 9.3 ± 0.7 10.3 111 0.36
Dy 10.2 ± 0.4 10.6 104 0.32
Er 6.5 ± 0.3 7.0 108 0.15
Ho 2.05 ± 0.17 2.22 108 0.11
Yb 7.4 ± 0.5 7.67 104 0.34
Lu 1.15 ± 0.09 1.11 97 0.19
Instrument: HR ICP-OES PlasmaQuant®
PQ 9000 Elite
Nebulizer Parallel path, PFA
Spray Chamber PTFE cyclonic, 50 mL
Torch Single slot quartz torch
Injector 2.0 mm alumina
Plasma Gas (L/min) 15
Aux Gas (L/min) 1.0
Neb Gas (L/min) 0. 5
Sample Rate (mL/min) 1.5
Plasma view axial](https://image.slidesharecdn.com/sampledissolution-190312161906/75/Sample-Dissolution-19-2048.jpg)