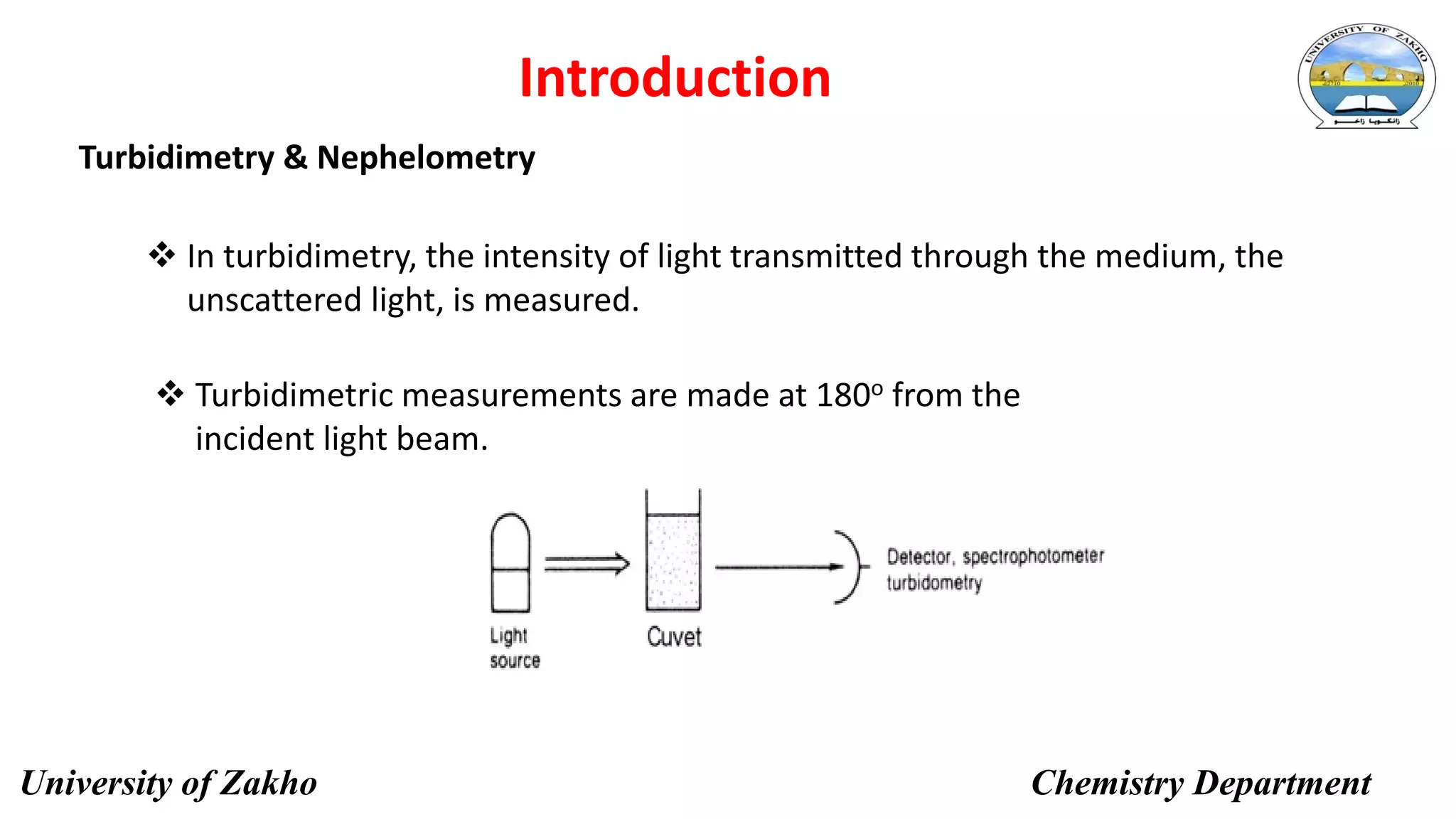
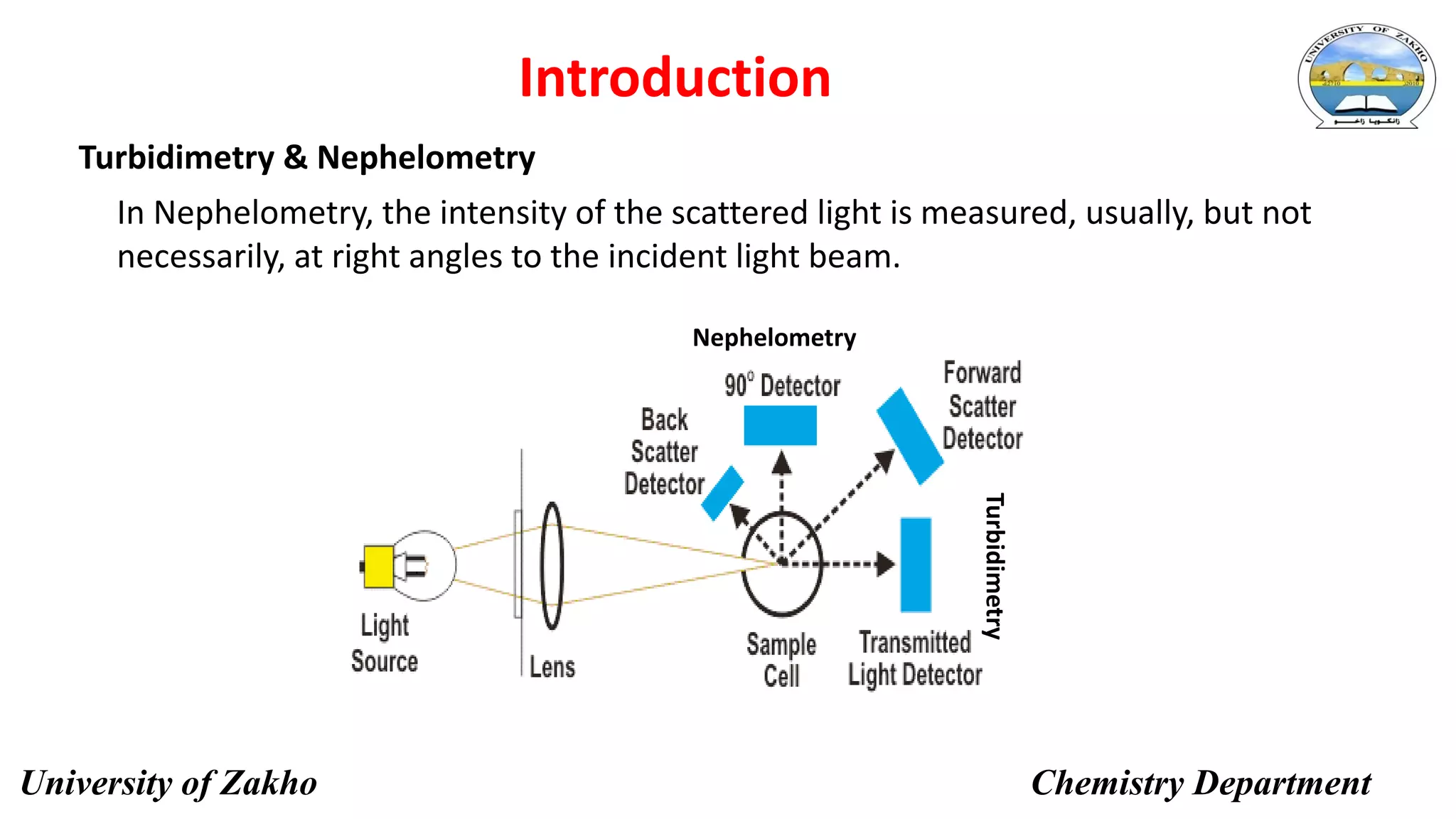
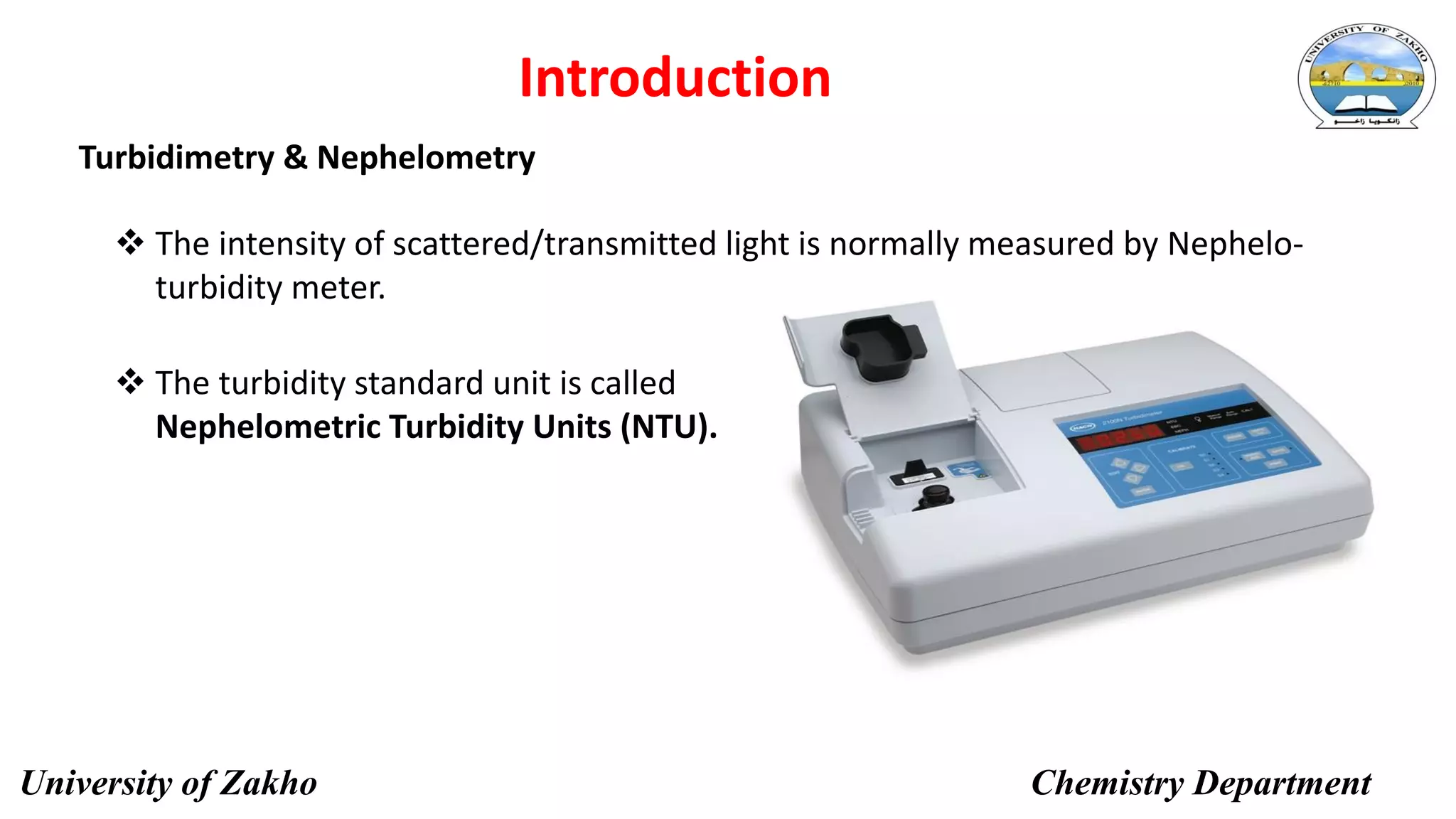
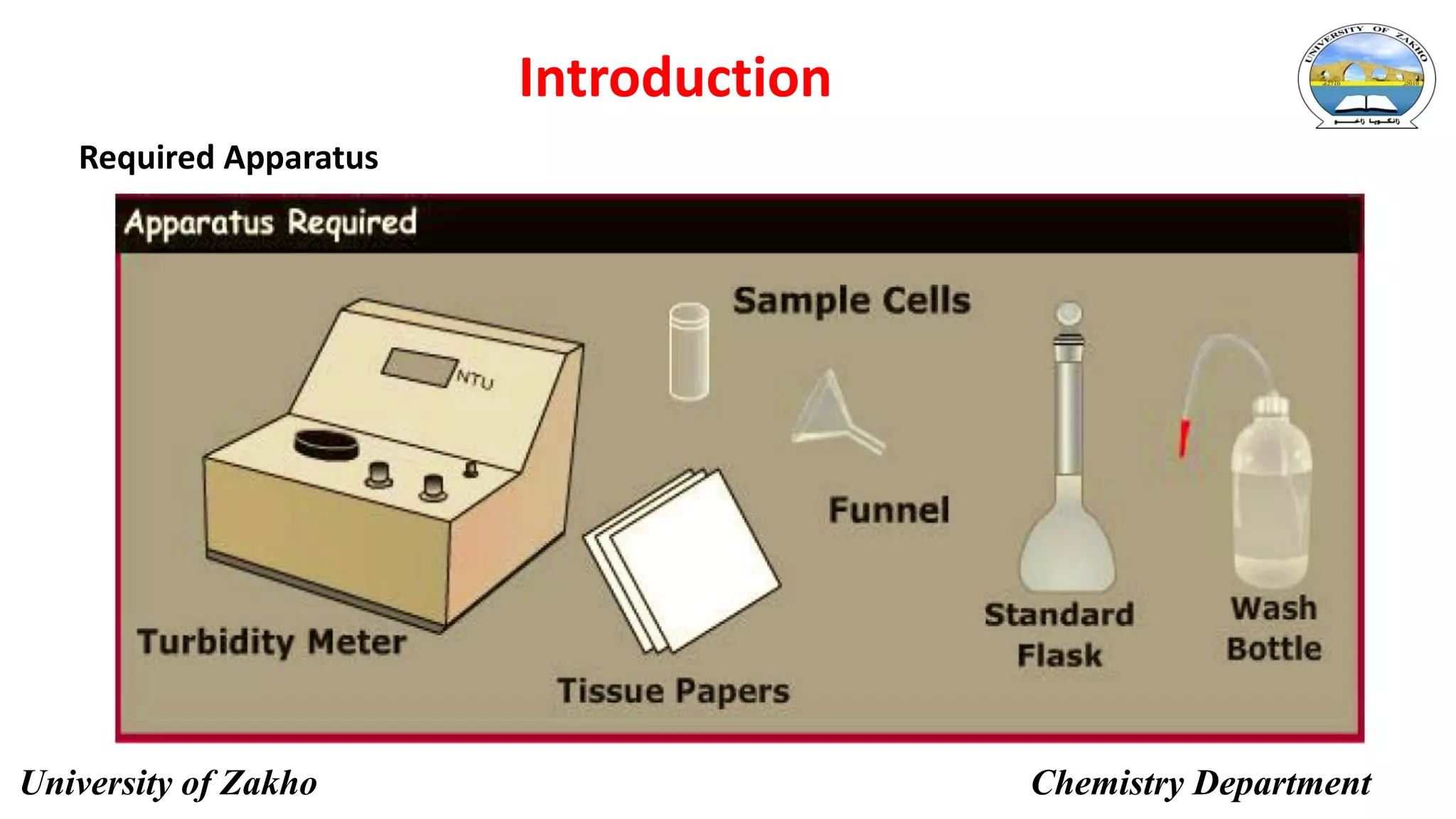
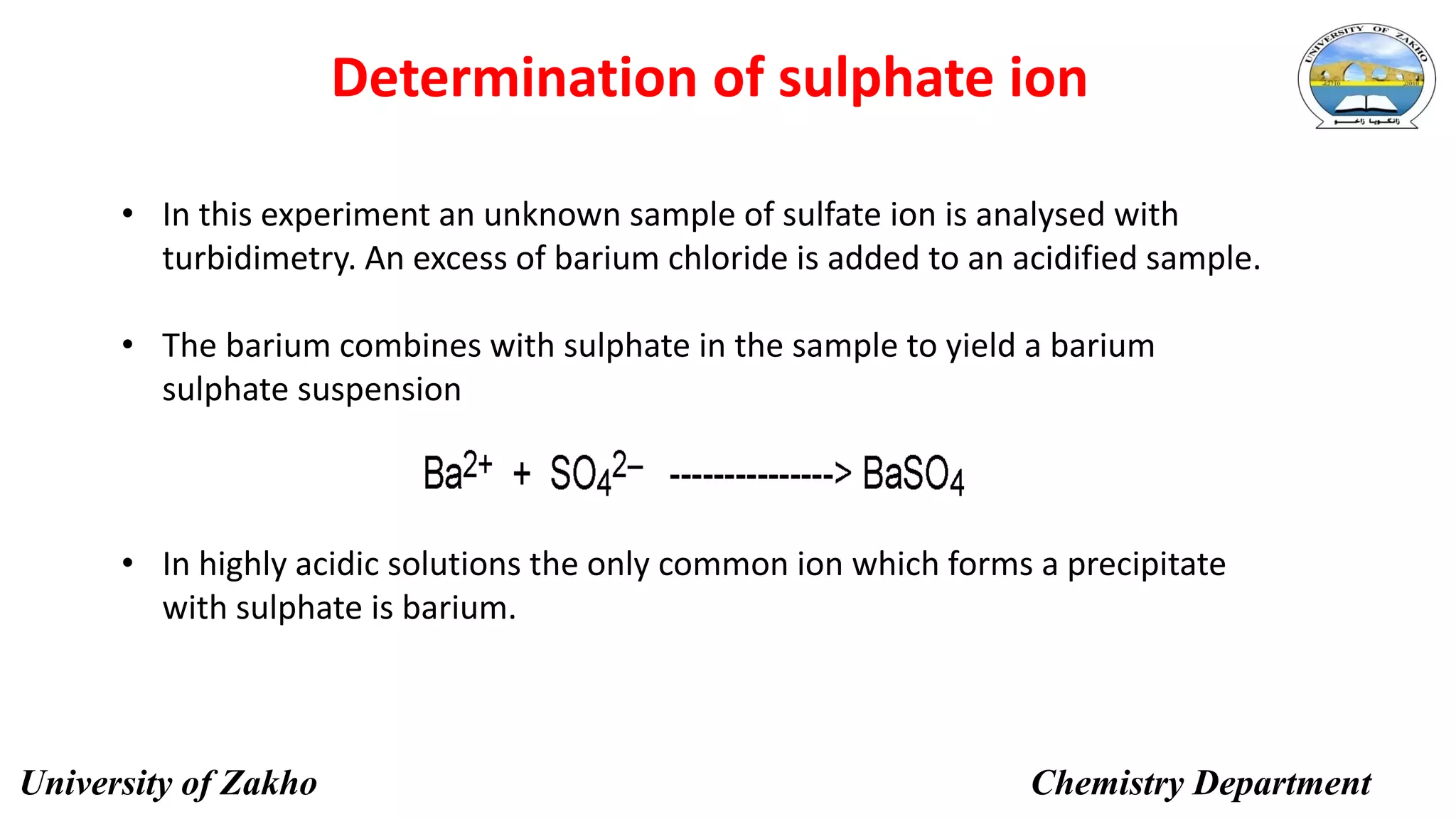
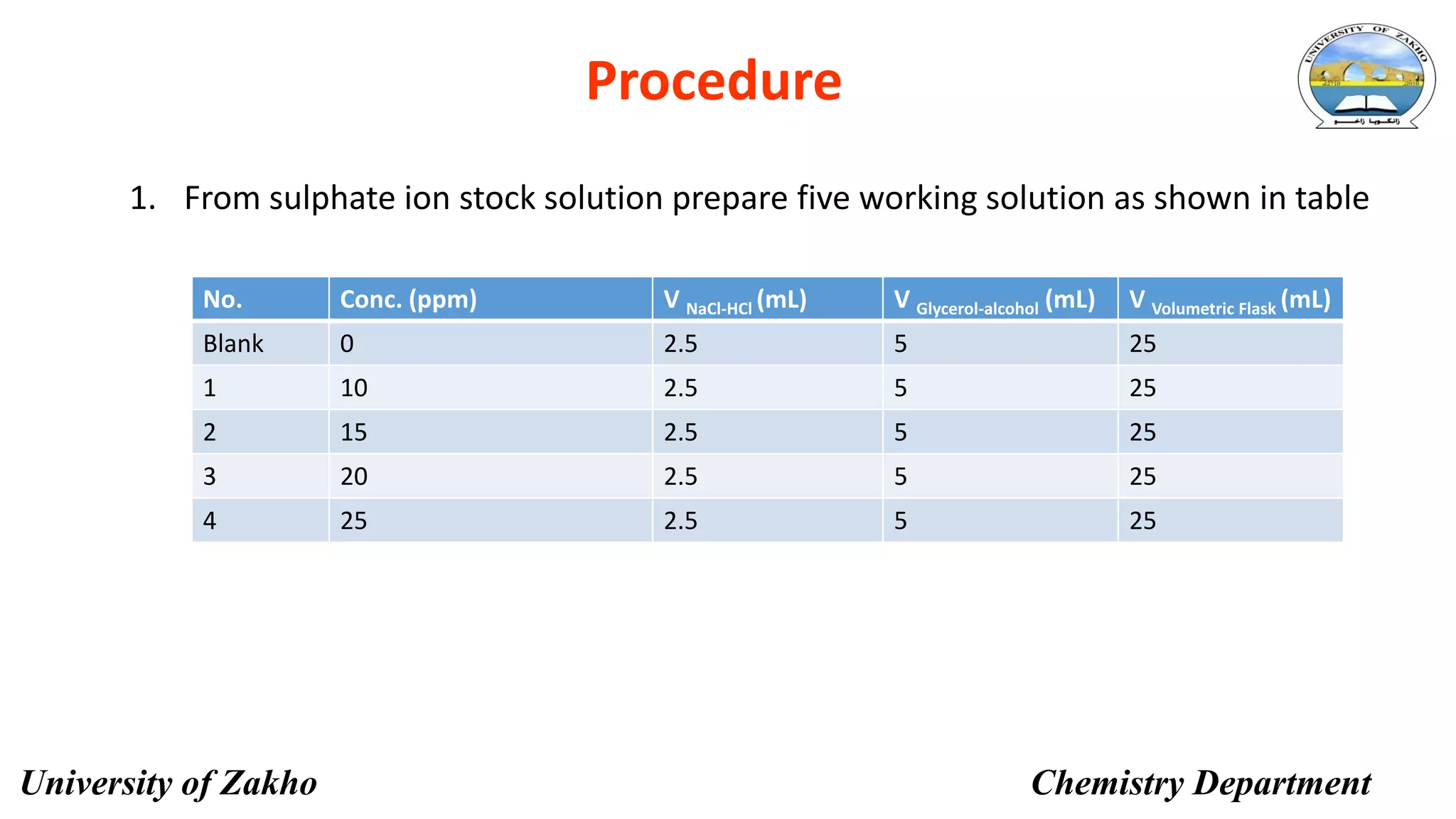
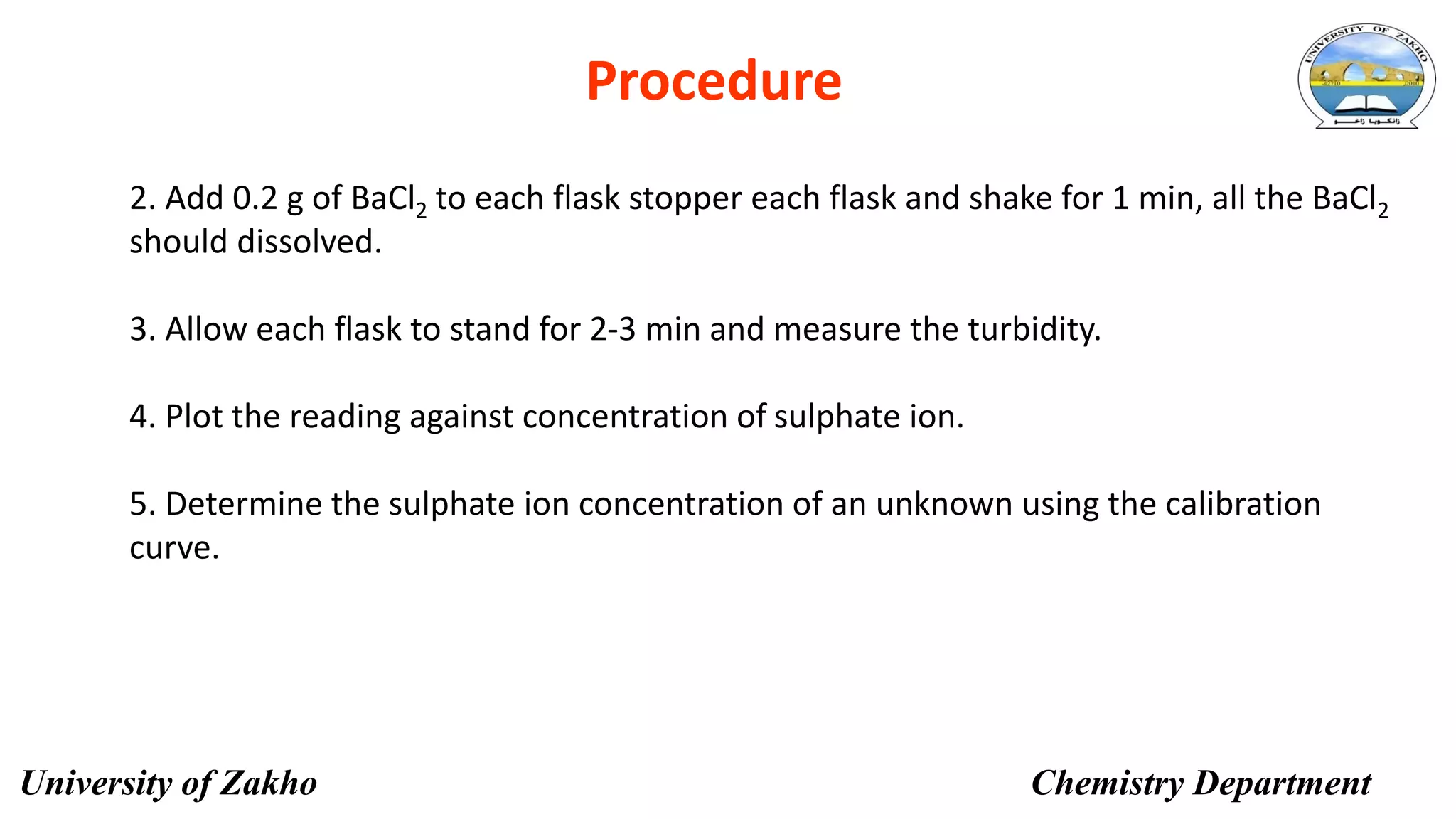
This document describes a procedure to determine the concentration of sulfate ions (SO42-) in an unknown solution using nephelo-turbidity meter. Barium chloride is added to acidified samples containing varying concentrations of sulfate ions to form a barium sulfate suspension. The turbidity of each sample is then measured and a calibration curve is prepared by plotting turbidity readings against sulfate ion concentration. This curve is then used to determine the concentration of sulfate ions in an unknown sample based on its turbidity reading.